METALS: NICKEL
Nickel, which is relatively rare in the Earth's crust (0.008%), is
usually found as a sulfide (e.g., in the ore millerite). This element
is a typical transition metal, as evinced by its high melting point
(1535°C), high density (8.91 g cm-³), variable oxidation states [e.g.,
Ni(II) and Ni(III)], formation of coloured compounds (which are often
green), and catalytic activity (e.g., it is used in the hydrogenation
and dehydrogenation of organic compounds).
[.. K > Cs > Ca > Na > Mg > Al > Fe > Ni > Sn > (H) > Cu > Hg > Ag ..]
1. Suggest how nickel can be extracted from nickel(II) oxide, which is
obtained by roasting its sulfides in air. _____________________________
_______________________________________________________________________
[1]
2. Perhaps coincidentally, the thermal stabilities of nitrates appear
to parallel the reactivity series. For example, mercury(II) nitrate
decomposes on very gentle heating, to give a silvery liquid, nitrogen
dioxide, and dioxygen: whereas, nickel(II) nitrate decomposes only on
moderately strong heating, to give a black solid, nitrogen dioxide, and
dioxygen: and, typical of Group 1 nitrates, caesium nitrate decomposes
only on very strong heating, to give a pale-yellow solid and dioxygen.
(a) Construct the symbol equation for each of these decompositions.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
(b) The volume (V1) of one mole of any gas at room temperature (25°C =
298 K; T1) and pressure (100 kPa; P1) is 24000 cm³; furthermore, the
following relationship holds true for gases:
P1 × V1 P2 × V2
¾¾¾¾¾ = ¾¾¾¾¾
T1 T2
Determine the volume (V2) of nitrogen dioxide, at room temperature and
low pressure (5 kPa; P2), obtained from the thermal decomposition of
3.66 g of nickel(II) nitrate - as follows.
Calculate the molar mass of nickel(II) nitrate. _______________________
_______________________________________________________________________
Calculate the number of moles of nickel(II) nitrate in 3.66 g of the
compound. _____________________________________________________________
Using the symbol equation, determine the number of moles of nitrogen
dioxide obtained from this number of moles of nickel(II) nitrate. _____
_______________________________________________________________________
Calculate the volume (V1) of gas obtained at room temperature (T1) and
pressure (P1). ________________________________________________________
And finally, using the above relationship, calculate the volume (V2) of
gas at the decreased pressure (P2). ___________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[9]
3. When powdered nickel is added to aqueous mercury(II) nitrate, the
grey solid rapidly dissolves, the colourless solution changes to green,
a silvery liquid forms, and the temperature of the solution increases.
Construct the net ionic equation for this redox reaction, complete with
a qualitative indication of the heat energy change. ___________________
_______________________________________________________________________
[3]
4. Suggest one reason why aqueous solutions of metal nitrates should
not be discharged into the environment. _______________________________
_______________________________________________________________________
[1]
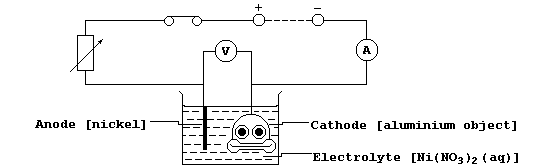
5. Shown below is a diagram of an electrolytic cell used to nickel-
plate an aluminium object.
[Q = n × z × F and Q = I × t, where: Q, measured in coulombs (C), is
the quantity of electricity; n is the number of moles of substance
evolved at the electrode; z is the charge on the ion; F is a constant,
with a value of 96500 C mol-¹; I, measured in amps (A), is the current;
and t, measured in seconds (s), is the time.]
(a) The mass (m) of the object increased by 0.383 g in 20 minutes.
Write an ionic equation for the reaction which occurs at the cathode.
_______________________________________________________________________
Calculate the number of moles (n) of nickel deposited at the cathode.
_______________________________________________________________________
Calculate the quantity of electricity (Q) required to deposit this
number of moles. ______________________________________________________
And finally, calculate the current (I) that flowed in the circuit. ____
_______________________________________________________________________
[7]
(b) State and explain what would be observed for each of the following,
if the polarities of the above circuit were reversed.
Nickel strip __________________________________________________________
_______________________________________________________________________
Aluminium object ______________________________________________________
_______________________________________________________________________
Electrolyte ___________________________________________________________
_______________________________________________________________________
[6]
6. Nickel's use as a catalyst is exemplified by the dehydrogenation of
ethylbenzene to phenylethene. [This alkene, better known as styrene, is
the monomer in the manufacture of poly(phenylethene).]
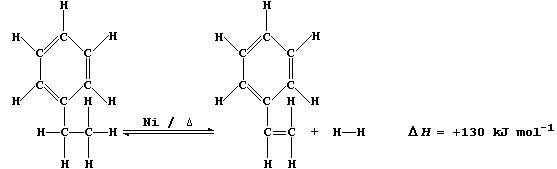
Suggest and explain two advantages in using a high temperature in the
above reaction. _______________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
Dr. R. Peters Next Contents' List
