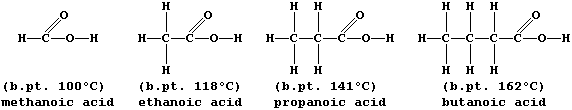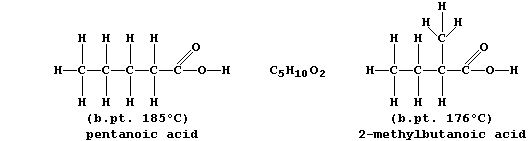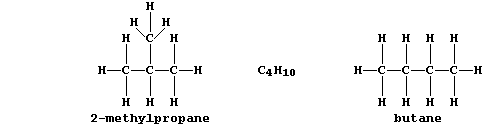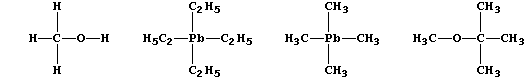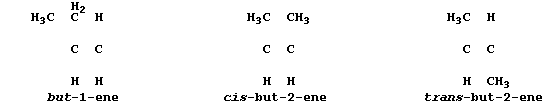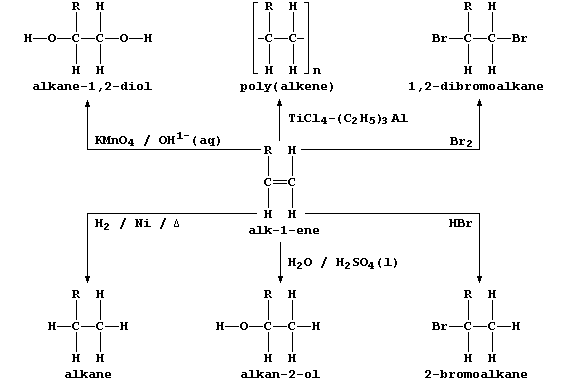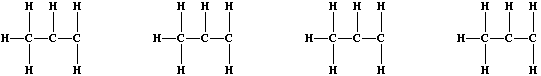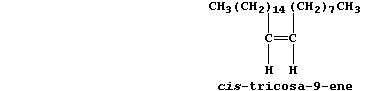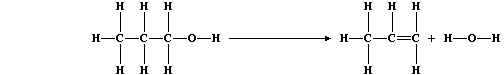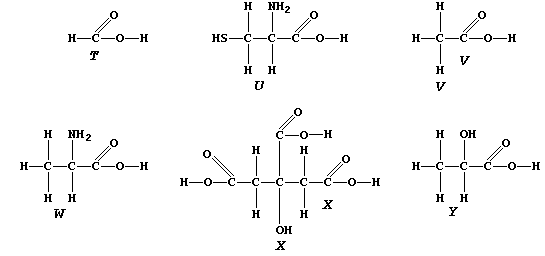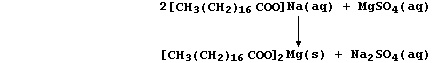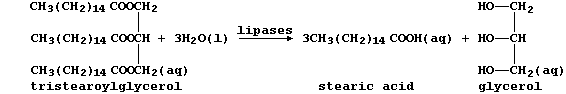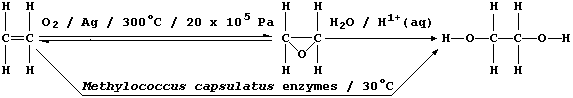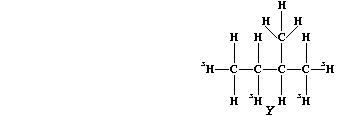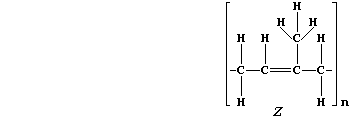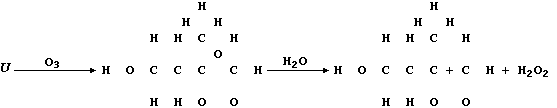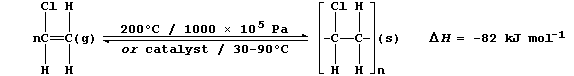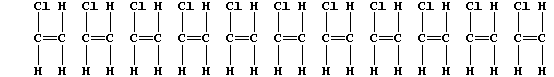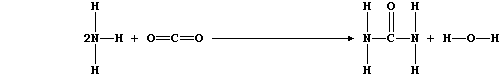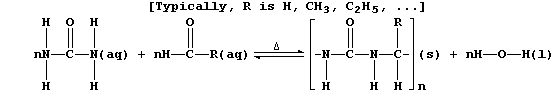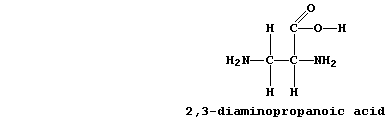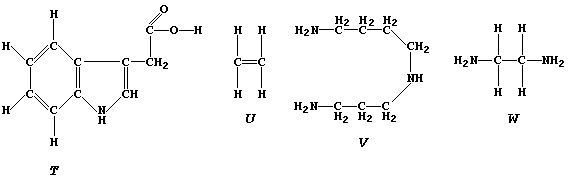AUFBAU1 [ORGANIC CHEMISTRY]
[01] INTRODUCTION - DIVERSITY / NOMENCLATURE
[02] INTRODUCTION - PETROLEUM / REACTION TYPES
[03] ALKANES
[04] ALKENES
[05] ALCOHOLS
[06] CARBOXYLIC ACIDS
[07] BIOTECHNOLOGY
[08] CATALYTIC HYDROGENATION
[09] ISOPRENE
[10] POLY(CHLOROETHENE)
[11] UREA
[12] BIOCIDES
[13] DETERGENTS
[14] MALE CONTRACEPTIVES
ORGANIC CHEMISTRY: INTRODUCTION - DIVERSITY
Carbon has an unparalleled ability to form stable chains, rings, inter-
connected rings, and a wide range of single and multiple bonds (e.g.,
C=C, C=N, C=O, C=S, CºC, and CºN). Accordingly, despite carbon being
only the 17th most abundant element in the Earth's crust (0.09%), it is
not surprising that organic compounds are ubiquitous and so numerous.
Certainly, the extraordinary diversity of living organisms is directly
attributable to the facility of carbon atoms to form large and complex
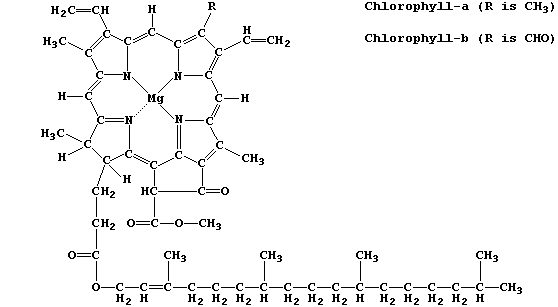
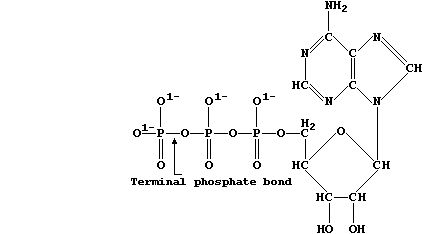
structures, as exemplified by chlorophyll-a and adenosine triphosphate
(ATP) - two of the most important molecules in the biosphere. [See, in
particular, P. W. Atkins, Molecules, Scientific American Library, New
York, 1987; this book is absolutely stunning or, using the author's own
felicitous words, "... a book for occasional delectation."]
Chlorophyll-a is the commonest photosynthetic pigment; its function is
to absorb light energy and transduce it into chemical energy.
Adenosine triphosphate is the immediate source of chemical energy for
all living organisms; the energy required for the endergonic processes
in cells, such as active transport and biosynthesis, is released via
enzymic hydrolysis of ATP's terminal phosphate bond.
ORGANIC CHEMISTRY: INTRODUCTION - NOMENCLATURE
The systematic name of an organic compound is divided into two parts:
one part indicates the chain length, ring size, or type of ring, and
the other indicates the functional group(s). A functional group is an
atom or group of atoms bonded to the carbon skeleton, and its reactions
will often determine the chemical properties of the whole compound.
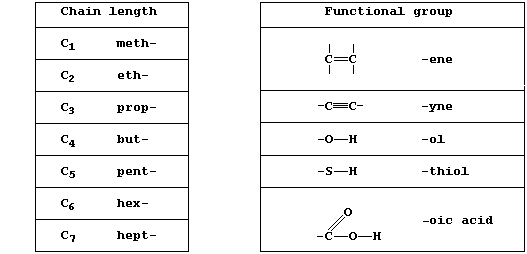
[Note, these two Tables present only a few of the prefixes and
suffixes used in the systematic naming of organic compounds. ]
A group of compounds are said to belong to the same homologous series
if they have the same functional group but different chain lengths or
ring sizes. Within a series, homologues usually show similar chemical
properties but different physical properties; e.g., all carboxylic
acids are neutralized by alkalis,
but their boiling points steadily increase with increasing molar mass:
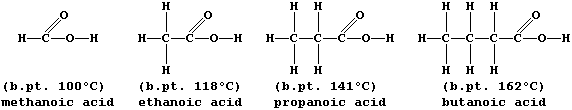
The molecular formulae of adjacent members of a series differ by CH2,
so each homologous series can be represented by a general formula (this
is CnH2nO2 for chain carboxylic acids). Finally, there are compounds
with the same molecular formula but different structural formulae -
these are known as isomers.
ORGANIC CHEMISTRY: INTRODUCTION - PETROLEUM
Petroleum, which was formed over millions of years by the compaction
of dead organisms and the anaerobic decomposition of their biological
molecules, is usually found trapped beneath impermeable cap rock and
above a lower dome of sedimentary rock.
This oil, a non-renewable 'fossil fuel', is a mixture of solid, liquid,
and gaseous hydrocarbons (mostly alkanes). In refineries, crude oil is
fractionally distilled - a physical process which separates the various
components on the basis of their different boiling points:
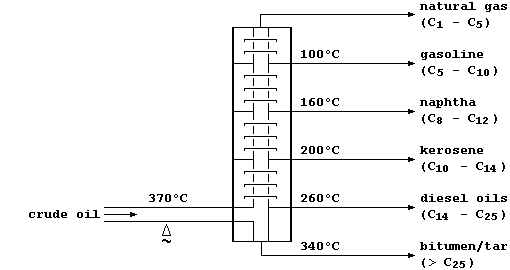
Some of these hydrocarbon fractions are used directly; e.g., as fuels
(natural gas), or to manufacture petrol (gasoline), or to surface roads
(tar). But, as this double bar-graph exemplifies, the demand for these
fractions rarely matches their relative amounts in typical crude oil.
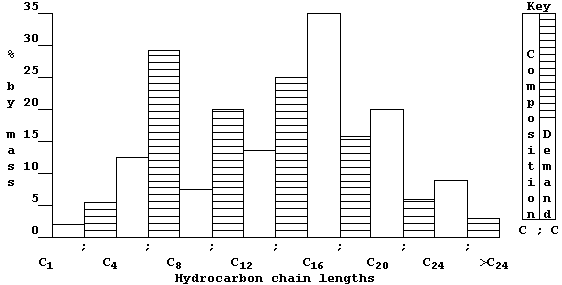
So, to overcome this economic problem, industry takes certain fractions
and subjects them to catalytic cracking - a chemical process whereby
large molecules are broken down by heat and by catalysis into smaller
(and more useful) molecules; e.g.,
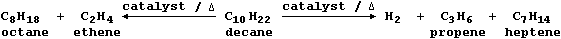
Mixtures of cracked products are separated by fractional distillation,
and the fractions used as fuels, solvents, or synthetic intermediates.
ORGANIC CHEMISTRY: INTRODUCTION - REACTION TYPES
The biological and industrial importance of organic compounds, together
with their structural diversity, has resulted in the development of
hundreds of synthetic methods. Happily, most of these can be classified
into just eight reaction types:
Addition (A), Condensation (C), Elimination (E), Hydrolysis (H),
Isomerization (I), Oxidation (O), Reduction (R), and Substitution (S).
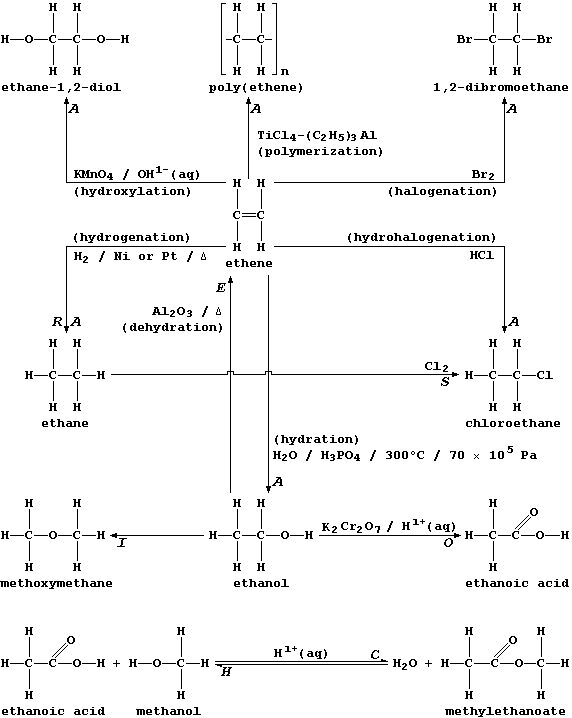
Two points are worth keeping in mind when considering the exemplars of
each reaction type shown above. First, more than one description can be
in common use for a given type; e.g., what is, formally, the addition
of hydrogen to ethene will also be described as the reduction or the
hydrogenation or the saturation of ethene. And second, each type can be
usefully viewed as one of a 'pair', as exemplified explicitly above by
addition-elimination and by condensation-hydrolysis.
ORGANIC CHEMISTRY: ALKANES
All chain alkanes belong to an homologous series which has the general
formula CnH2n+2; the Table shows some data for typical homologues. In
industry, most of these saturated hydrocarbons are usually obtained by
the fractional distillation of either petroleum oil or the products of
cracking; they are used mainly as fuels and as industrial solvents.
Systematic name |
Molecular formula
(state at 25°C) |
Boiling
pt. / °C |
Heat of combustion
(DH) / kJ mol-¹ |
Ethane |
C2H6 (g) |
-89 |
-1560 |
Propane |
C3H8 (g) |
-42 |
-2220 |
2-Methylpropane |
C4H10 (g) |
-11.5 |
-2875 |
Butane |
C4H10 (g) |
-0.5 |
-2880 |
Octane |
C8H18 (l) |
+126 |
-5520 |
Eicosane |
C20H42 (s) |
+343 |
-13440 |
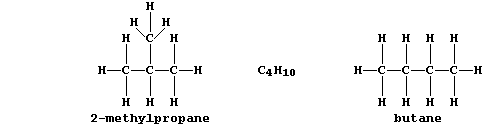
1. The female tiger moth (Homomelia imaculata) signifies its presence
to the males by secreting minute quantities of a sex attractant. This
example of an insect 'pheromone' is a 2-methyl branched-chain alkane
with a molecular formula of C18H38; the straight-chain isomer does not
elicit a response. Noting the structural difference between butane and
2-methylpropane, draw the structural formula of this pheromone. _______
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
2. Natural gas, which is mostly methane, is a non-renewable resource.
However, fermentation of sewage gives a mixture of methane and carbon
dioxide known as 'biogas'; the following symbol equation summarizes one
biological process that occurs in fermenters and in landfill sites:
Methanobacteria enzymes
C6H12O6(aq) —————————————————————® 3CH4(g) + 3CO2(g) -DE
(a) State briefly why both methane and carbon dioxide are considered to
be 'greenhouse' gases. ________________________________________________
_______________________________________________________________________
[1]
(b) Biogas can be used as a fuel, because some of its chemical energy
is transduced to 'high-grade' heat energy when the methane burns:
CH4(g) + 2O2(g) —————————————————————® CO2(g) + 2H2O(g) -DH
The heat of combustion for a straight-chain alkane can be calculated
from the linearly proportional relationship DH = k × n + c, where: k
is a proportionality constant, with a value of -660 kJ mol-¹; n is the
number of carbons; and c is a constant, with a value of -240 kJ mol-¹.
Use this relationship to calculate the heat of combustion (DH) for:
Methane (b.pt. -164°C) ________________________________________________
Pentane (b.pt. +36°C) _________________________________________________
[3]
3. Petrol is a blended mixture of several branched- and straight-chain
alkanes, including heptane [C7H16(l)], octane, and nonane [C9H20(l)].
The symbol equation for the complete combustion of gaseous heptane is:
2C7H16(g) + 22O2(g) —————————® 14CO2(g) + 16H2O(g)
Incomplete combustion will occur, however, if there is not sufficient
atmospheric oxygen available; e.g.,
2C7H16(g) + 15O2(g) —————————® 14CO(g) + 16H2O(g)
(a) Construct a symbol equation for the complete combustion of octane.
_______________________________________________________________________
[2]
(b) The combustion properties of petrol are improved by additives such
as methanol [CH3OH(l)], tetraethyllead [(C2H5)4Pb(l)], tetramethyllead
[(CH3)4Pb(l)], and methoxy-2-methylpropane [CH3OC(CH3)3(l)].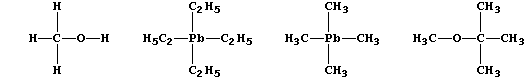
Construct a symbol equation for the synthesis of tetraethyllead, which
is manufactured by heating a mixture of sodium, lead, and chloroethane.
_______________________________________________________________________
[2]
Suggest a suitable solvent for tetraethyllead; this covalent compound
is immiscible with water. _____________________________________________
[1]
Tetraethyllead burns to form lead(IV) oxide, carbon dioxide, and steam.
Construct the symbol equation for this combustion reaction. ___________
_______________________________________________________________________
[2]
Suggest one advantage in using methoxy-2-methylpropane as an additive.
_______________________________________________________________________
[1]
(c) Pollutants emitted from a vehicle exhaust include carbon monoxide,
nitrogen oxides, and unburned hydrocarbons; these are converted into
safer emissions if the exhaust is fitted with a 'catalytic convertor':
Pt-Rh / D
2CO(g) + 2NO(g) —————————® CO2(g) + N2(g)
Describe one biological effect of carbon monoxide. ____________________
_______________________________________________________________________
_______________________________________________________________________
[2]
State three effects of nitrogen oxide pollutants. _____________________
_______________________________________________________________________
_______________________________________________________________________
[3]
Vehicles with convertors must use unleaded-petrol to minimize 'catalyst
poisoning'. Explain how lead compounds inhibit such catalysts. ________
_______________________________________________________________________
_______________________________________________________________________
[2]
4. Candlewax is usually a mixture of organic compounds from different
homologous series, including alkanes such as eicosane and triacontane
(C30H62). Suggest one technique which could be used to show that wax is
not a pure compound. __________________________________________________
[1]
Products formed from the incomplete combustion of wax include soot and
carbon monoxide. Soot is largely a non-crystalline (or amorphous) form
of which element? _____________________________________________________
[1]
ORGANIC CHEMISTRY: ALKENES
All chain alkenes belong to an homologous series which has the general
formula CnH2n; and all - except ethene and propene - can occur in two
or more isomeric forms (as evinced by molecular models). In industry,
many of these unsaturated hydrocarbons are obtained by the fractional
distillation of products formed by cracking petroleum oil fractions.
Alkenes are used as synthetic intermediates; this use, as well as their
versatility, is attributable to the wide variety of reagents which add
across the reactive double bond present in every alkene.
1. Complete these structural formulae to show the three alkene isomers
which have the molecular formula of C4H8.
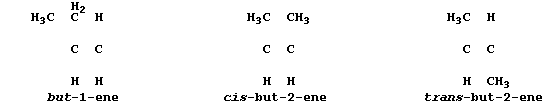
[2]
2. This general scheme summarizes some typical addition reactions of
alk-1-enes, where R may be CH3, C2H5, C3H7, C4H9, ...
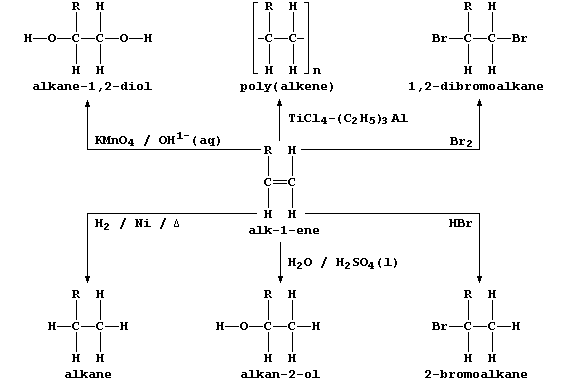
In principle, and often so in practice, the addition of a reagent to an
alkene can result in the formation of isomers; e.g., the addition of
water and of hydrogen bromide to but-1-ene, R = C2H5, could also give
butan-1-ol and 1-bromobutane, respectively. Draw the full structural
formulae of 1-bromobutane and 2-bromobutane. __________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
3. Isomerism can be considered to represent, Janus-like, 'the beauty
and the bane' of Chemistry ... Thus, on the one hand, isomers often
show important differences in chemical and biological properties; e.g.,
acidified potassium dichromate(VI) oxidizes butan-1-ol, a highly toxic
industrial solvent, but has no effect on ethoxyethane, a sparingly used
anaesthetic popularly known as 'ether'.
However, on the other hand, mixtures of isomers require separation by
physical methods that are often costly in terms of time and/or energy.
Name three physical methods used to separate mixtures. ________________
_______________________________________________________________________
[3]
4. A number of solvents are manufactured by the addition reactions of
halogens with alkenes; e.g., dichlorine reacts with all three butenes
to form dichlorobutanes. Recalling that naturally occurring chlorine
consists of two isotopes, (Cl-35 and Cl-37), complete these structural
formulae to show the four isotopic variants of 1,2-dichloropropane.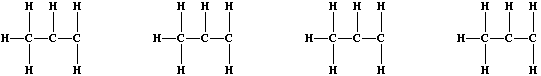
[2]
5. Polymerization is a process whereby thousands of monomer molecules
react to form one polymer molecule; and, as indicated by the general
equation below, alkene monomers undergo addition polymerization:

Calculate the molar mass of the monomeric unit in Perspex, correctly
named as poly(2-methylpropenoate); i.e., R° = CH3, R¹ = H, R² = CO2CH3
and R³ = H. ___________________________________________________________
[2]
6. The major sex pheromone of the female housefly (Musca domestica) is
cis-tricosa-9-ene; its primary function seems to be sexual stimulation
of the males.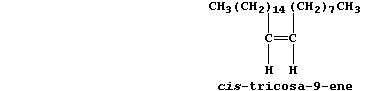
Determine the molecular and empirical formulae of cis-tricosa-9-ene.
_______________________________________________________________________
Name one isomer which would be less likely to behave as a pheromone in
this species. _________________________________________________________
[3]
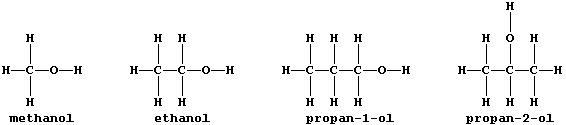
ORGANIC CHEMISTRY: ALCOHOLS
All alcohols contain the hydroxyl functional group, -O-H, and many
belong to an homologous series which has the general formula CnH2n+1OH;
this first Table shows some data for five straight-chain homologues.
Systematic name |
Condensed
formula |
Boiling pt.
/ °C |
Density
/ g cm-³ |
Heat of Combustion
(DH) / kJ mol-¹ |
Methanol |
CH3OH |
65 |
0.791 |
-715 |
Ethanol |
C2H5OH |
78 |
0.798 |
-1370 |
Propan-1-ol |
C3H7OH |
97 |
0.804 |
-2010 |
Butan-1-ol |
C4H9OH |
118 |
0.810 |
-2650 |
Pentan-1-ol |
C5H11OH |
138 |
0.815 |
-3320 |
1. Use the data in this Table to state, as precisely as possible, the
pattern between molecular formula and the:
Boiling point _________________________________________________________
_______________________________________________________________________
Heat of combustion ____________________________________________________
_______________________________________________________________________
[3]
2. Extrapolate these data to predict the boiling point and the heat of
combustion for hexan-1-ol. ____________________________________________
[2]
3. Why are propan-1-ol and propan-2-ol considered to be isomeric? ____
_______________________________________________________________________
[1]
4. The symbol equations for the combustion of methanol and propan-1-ol
are as follows:
2CH3OH(g) + 3O2(g) —————————————————————® 2CO2(g) + 4H2O(g)
2C3H7OH(g) + 9O2(g) —————————————————————® 6CO2(g) + 8H2O(g)
(a) Construct the symbol equation for the combustion of pentan-1-ol.
_______________________________________________________________________
[2]
(b) How many moles of air are required for the (complete) combustion of
1.0 mole of pentan-1-ol? ______________________________________________
_______________________________________________________________________
[2]
(c) Suggest two products, other than carbon dioxide and water, that
might be formed when pentan-1-ol is burnt in a limited supply of air.
_______________________________________________________________________
[2]
(d) Calculate the energy released from the combustion of one molecule
of pentan-1-ol (1 mole = 6.022 × 10²³ particles). _____________________
_______________________________________________________________________
[2]
5. Apart from their use as fuels and as synthetic intermediates,
alcohols are used extensively as non-aqueous solvents; in particular,
and in contrast to water, they dissolve a wide range of covalently
bonded solutes. Although ethanol is drunk socially in beverages (e.g.,
beers and spirits), most alcohols are extremely toxic. Name two human
organs which are damaged by solvent abuse. ____________________________
[2]
6. In industry, large quantities of alkenes are obtained via the
catalytic cracking of petroleum oil fractions. However, the most
convenient method of preparing a small quantity of any alkene is by
catalytic dehydration of the corresponding alcohol; e.g., propene can
be prepared from propan-1-ol using the apparatus shown in the diagram.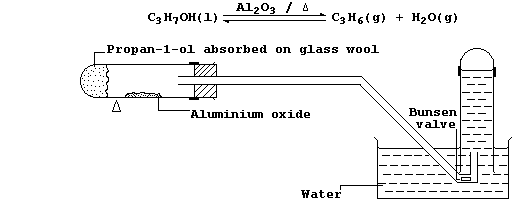
(a) Alcohols can be dehydrated by heating with concentrated sulfuric
acid. Suggest and explain one reason why this dehydrating agent is less
commonly used. ________________________________________________________
_______________________________________________________________________
[2]
(b) Explain the purpose of the Bunsen valve. __________________________
_______________________________________________________________________
[2]
(c) Suggest what would be observed if, in the above experiment, the
water was replaced with alkaline potassium manganate(VII). ____________
_______________________________________________________________________
_______________________________________________________________________
[2]
(d) The equation for this dehydration reaction can be written as: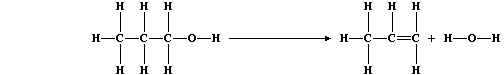
Complete this second Table using these bond energies (in kJ mol-¹):
C-C (346); C-O (360); C-H (413); O-H (463); and C=C (610).
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
7 C-H |
|
6 C-H |
|
2 C-C |
|
1 C-C |
|
1 C-O |
|
1 C=C |
|
1 O-H |
|
2 O-H |
|
Total = |
Total = |
[4]
Calculate the heat energy change (DH) for the above reaction. _________
_______________________________________________________________________
[2]
(e) State and explain, using Le Chatelier's Principle, what would be
the effect of high pressure on this dehydration reaction. _____________
_______________________________________________________________________
_______________________________________________________________________
[2]
ORGANIC CHEMISTRY: CARBOXYLIC ACIDS
The carboxylic acid functional group, -COOH, is present in a variety
of common chemical substances. This Table provides some information on
six such compounds; their structural formulae are shown below.
|
Systematic name / Common name |
Natural Occurrence |
|
Methanoic / Formic acid |
Wood ants (Formica rufa) |
|
2-Hydroxypropanoic / Lactic acid |
Sour milk / Active muscles |
|
2-Hydroxypropane-1,2,3-tricarboxylic
/ Citric acid |
Citrus fruits; e.g., sweet
oranges (Citrus sinensis) |
|
Ethanoic / Acetic acid |
Vinegar |
W |
2-Aminopropanoic acid / Alanine |
Via hydrolysis of proteins |
|
2-Amino-3-mercaptopropanoic acid /
Cysteine |
Via hydrolysis of proteins |
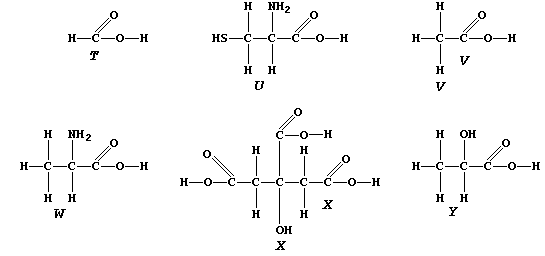
1. Complete the Table above, by reference to the systematic names and
structural formulae.
[5]
2. In contrast to 'mineral' acids [e.g., HCl(aq)], organic acids only
partially dissociate when dissolved in water; e.g., the position of
equilibrium lies far to the left in an aqueous solution of lactic acid: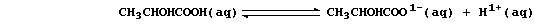
Although the lower concentration of hydrogen ions means that organic
acids react more slowly than mineral acids, reactions occur because -
as predicted by Le Chatelier's Principle - each equilibrium continually
'shifts to the right' as hydrogen ions are removed by reaction with
another reactant. Construct a symbol equation for the reaction between:
Magnesium and aqueous ethanoic acid ___________________________________
_______________________________________________________________________
Aqueous solutions of lactic acid and sodium hydroxide _________________
_______________________________________________________________________
Aqueous solutions of methanoic acid and potassium carbonate ___________
_______________________________________________________________________
Aqueous ethanoic acid and copper(II) oxide ____________________________
_______________________________________________________________________
[8]
Complete the following description of one method of preparing crystals
of hydrated copper(II) ethanoate (which has been used as a fungicide
and as an insecticide). "Wear _______ glasses. To 25 cm³ of warm - but
not boiling - ethanoic acid (1.0 mol dm-³), add copper(II) carbonate in
small portions until no more of this pale-green solid __________ (and
there is no more fizzing). Filter the mixture, partially __________ the
filtrate using a boiling water-bath, and then leave the dark-green
solution to crystallize at room temperature in a labelled petri dish."
[3]
3. One synthetic route to carboxylic acids involves oxidation of the
corresponding alcohol with acidified potassium dichromate(VI).
(a) When Acetobacter aceti bacteria use the ethanol present in wine as
a respiratory substrate, in order to biosynthesize ATP via aerobic
respiration, they excrete (sour-tasting) ethanoic acid:
Acetobacter aceti enzymes
C2H5OH(aq) + O2(g) ————————————————® CH3COOH(aq) + H2O(l) -DE
For the large-scale manufacture of ethanoic acid, suggest one advantage
in using a biotechnological process with these (immobilized) bacteria.
_______________________________________________________________________
[1]
Suggest the meaning of the term '- DE'. _______________________________
_______________________________________________________________________
[1]
(b) When Lactobacillus bacteria use the lactose present in milk as a
respiratory substrate, in order to biosynthesize ATP via anaerobic
respiration, they excrete (similarly sour-tasting) lactic acid:
Lactobacillus enzymes
C12H22O11(aq) + H2O(l) ————————————————® 4CH3CHOHCOOH(aq) -DE
Lactic acid is also formed, from glucose using different enzymes, when
a mammalian muscle carries out anaerobic respiration. The acid is toxic
to cells, and so must be oxidized back to glucose in the liver. How is
oxygen gas transported in the blood of a mammal? ______________________
[1]
4. This second Table provides some information on four of the main
naturally occurring, long-chain, saturated carboxylic acids (commonly
referred to as fatty acids because they were first isolated from fats).
Systematic name |
Common Source |
Condensed formula |
Tetradecanoic acid |
Coconuts (Cocus nucifera) |
CH3(CH2)12COOH |
Hexadecanoic acid |
Oil palms (Elaesis guineensis) |
CH3(CH2)14COOH |
Octadecanoic acid |
Cocoa seeds (Theobroma cacao) |
CH3(CH2)16COOH |
Eicosanoic acid |
Groundnuts (Arachis hypogea) |
CH3(CH2)18COOH |
(a) A typical soap is the sodium salt of octadecanoic acid, better
known as stearic acid, whose anions react with Group 2 cations present
in hard water to form a precipitate of 'scum'; e.g.,
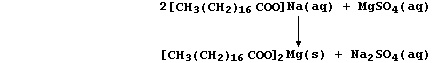
Noting that the spectator ions are aqueous sodium and aqueous sulfate,
construct the net ionic equation for this precipitation reaction. _____
_______________________________________________________________________
[2]
(b) The condensation of three fatty acids and glycerol, more correctly
known as propane-1,2,3-triol, results in a triglyceride. Fats and oils,
which are usually mixtures of triglycerides, are hydrolyzed in mammals
by lipases, as this scheme exemplifies: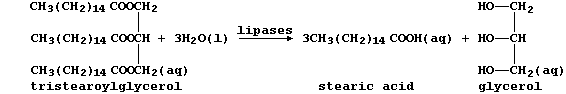
Several naturally occurring triglycerides contain unsaturated carbons.
Name one reagent which could show whether a fat or an oil was 'high in
polyunsaturates'. _____________________________________________________
[1]
ORGANIC CHEMISTRY: BIOTECHNOLOGY
Currently, in developed countries, most energy sources and vast numbers
of chemicals and manufactured products are derived from gas, oil, and
coal. To conserve these non-renewable fossil fuels, as well as to
reduce pollution, alternative sources of energy need to be harnessed.
Encouragingly, all of the major chemical companies have begun to apply
biotechnology; i.e., the industrial use of genes from micro-organisms
to synthesize products considered useful to Man. Chemicals which have
been made using biotechnology include benzylpenicillin (an antibiotic),
ethanol, ethane-1,2-diol, insulin (a hormone), poly(hydroxybutyrate)
(a biodegradable thermoplastic), rennin (an enzyme), and single-cell
protein (SCP; a food).
1. Ethanol is burnt in vehicle engines, instead of petrol, in several
developing countries, partly to reduce their dependence on (imported)
oil and partly as a conservation measure. This ethanol is obtained via
the fermentation of sucrose solutions with yeast; i.e.,
Saccharomyces enzymes / 35°C
C12H22O11(aq) + H2O(l) —————————————————————® 4C2H5OH(aq) + 4CO2(g) -DE
(a) Sugar cane (Saccharum officinarum) and sugar beet (Beta vulgaris)
are the main natural sources of sucrose; the structural formula of this
disaccharide is:
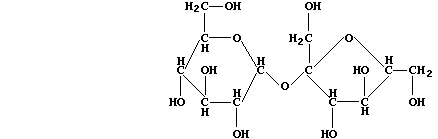
In order to hydrolyze sucrose, Saccharomyces species biosynthesize the
enzyme saccharase; this biological catalyst is roughly 10¹² times more
effective than the typical acid catalysts used in laboratories. State
the molecular formula of glucose and fructose, which are the (isomeric)
monosaccharides produced when this disaccharide is hydrolyzed. ________
[1]
(b) In anaerobic cellular respiration, with glucose as the respiratory
substrate, the term 'alcohol fermentation' strictly refers to the final
pair of eleven reactions (each catalyzed by a specific enzyme); though,
in practice, the term 'fermentation' is being used increasingly to
describe a wide variety of biotechnological processes. Explain why the
speed of fermentation is usually slower at 15°C than at 35°C. _________
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) The mixture at the end of fermentation is first filtered, to remove
suspended particles (in particular, the increased mass of yeast), and
then fractionally distilled, to separate the ethanol from the water.
State two major costs of either of these physical processes. __________
_______________________________________________________________________
[2]
(d) Construct the symbol equation for the combustion of ethanol. ______
_______________________________________________________________________
[2]
(e) Ethanol is usually manufactured in developed countries by catalytic
hydration of ethene. Construct the symbol equation for this reversible
reaction. _____________________________________________________________
_______________________________________________________________________
[2]
2. Ethane-1,2-diol, commonly known as ethylene glycol, is a liquid at
room temperature; it is used as a solvent, as a starting material for
a range of chemicals (e.g., polyesters and polyurethanes), and as an
'antifreeze' (e.g., in car radiators). In industry, this compound used
to be synthesized exclusively by a process which requires dioxygen
(from the air), a silver catalyst, and a high reaction temperature and
pressure: relatively recently, however, a fermentation process using
Methylococcus capsulatus bacteria has been developed.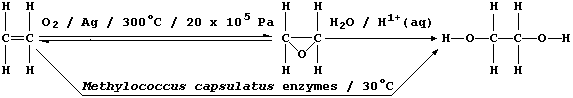
(a) State the empirical formula of ethane-1,2-diol. ___________________
Determine the general formula of the homologous series to which ethane-
1,2-diol belongs. _____________________________________________________
[2]
(b) In the industrial synthetic process, suggest one disadvantage in
using a silver catalyst. ______________________________________________
[1]
(c) Suggest one reason why higher temperatures are not used in the
fermentation process, apart from minimizing costs. ____________________
_______________________________________________________________________
[1]
(d) Suggest one disadvantage in using ethene as the starting material.
_______________________________________________________________________
[1]
3. Shown below are the structural formulae of water, ethanol, ethane-
1,2-diol, and propane-1,2,3-triol (glycerol). Rather atypically for
organic compounds, these three alcohols are miscible with water in all
proportions; their solubility is partially attributable to 'hydrogen
bonding' [i.e., the weak electrostatic attraction between the nucleus
of a bonded hydrogen atom and a 'lone-pair' of electrons of a bonded
non-hydrogen atom (typically, nitrogen or oxygen)].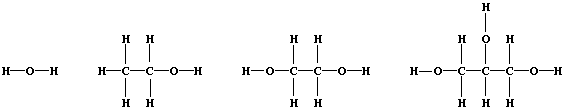
(a) Glycerol's main rôle in living organisms is as an intermediate in
the biosynthesis of fats and oils. However, certain organisms living in
cold habitats also incorporate glycerol into their cellular cytoplasm
to act as an antifreeze. Name one physical process which is slowed down
when biological cells are frozen. _____________________________________
[1]
(b) Glycerol, which is used extensively in the manufacture of solvents,
explosives, and polymers, is obtained by the hydrolysis of fats and
oils using either alkalis or lipases. Suggest one advantage in using
enzymes to effect these hydrolyses. ___________________________________
[1]
(c) Providing either a suitable micro-organism can be isolated or the
genes coding for the biosynthesis of glycerol can be identified, what
might be a suitable starting material to obtain this triol by
fermentation? _________________________________________________________
[1]
ORGANIC CHEMISTRY: CATALYTIC HYDROGENATION
Margarine and butter are both mixtures of fats, and so are useful
sources of chemical energy in the diet. Margarine, however, is much
lower in cholesterol; this steroid is required for homeostasis, because
it is both an essential component of cell membranes and an intermediate
in the biosynthesis of the steroid hormones: but, consistently high
concentrations of cholesterol circulating in the bloodstream appear to
increase the chances of contracting cardio-vascular diseases.
1. Margarine is made from vegetable oils which contain unsaturated
carbons. Hydrogenation of these oils, at 170°C using a nickel catalyst,
saturates the carbon chains; this raises the melting point of the oils
so that they become solid at room temperature. Prompted by the need to
minimize energy costs, manufacturers have considered the possibilities
of biotechnology. Recently, one company's research team have isolated
several enzymes from anaerobic micro-organisms living in the Amazonian
rainforest (a non-renewable resource). In qualitative studies, one of
these enzymes was shown to be effective in the hydrogenation of a wide
range of unsaturated compounds; and in quantitative studies, controlled
experiments were used to determine the speed of these hydrogenations.
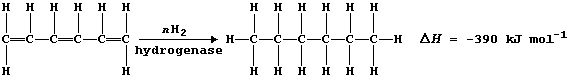
For example, the following hypothesis was examined: 'The speed (S) of
hydrogenating hexa-1,3,5-triene increases in direct proportion to the
amount (C) of hydrogenase enzyme "X"; i.e., S = k × C'; the Table shows
a summary of the chosen conditions and raw data.
Constants: hexa-1,3,5-triene (1.00 mole); volume of ethanol (1 dm³);
dihydrogen (excess); temperature (23°C); absence of light and dioxygen.
Amount (C) of "X"
hydrogenase / mmol |
0.00 |
0.50 |
1.00 |
1.00 |
1.00 |
1.50 |
2.00 |
Reaction time / s |
»3600 |
126 |
77 |
61 |
59 |
40 |
31 |
Reaction speed (S)
/ s-¹ |
0.000 |
|
|
|
|
|
|
(a) At room temperature (25°C) and pressure (100 kPa), what volume
of hydrogen gas is theoretically required for complete hydrogenation of
1.00 mole of hexa-1,3,5-triene? _______________________________________
[1]
(b) Suggest one reason why, in order to maintain a temperature of 23°C,
the reaction vessel needed to be externally cooled. ___________________
_______________________________________________________________________
[1]
(c) State the independent variable in the above investigation. ________
_______________________________________________________________________
[1]
(d) Calculate the missing values for the reaction speed, and insert
these data into the Table above.
[2]
(e) A value of a dependent variable which does not follow the general
pattern is referred to as an 'anomalous result'. In this investigation,
for example, the first reaction speed for C = 1.00 mmol appears
anomalous. Suggest one explanation for this anomaly. __________________
_______________________________________________________________________
[1]
(f) Label both axes, plot all seven data points, and then draw a best
straight line through as many points as is sensible.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
0.032_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0.024_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0.016_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0.008_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0.000_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | |
0 0.4 0.8 1.2 1.6 2.0 2.4
[4]
Determine the gradient of the graph; this value, 'k', is the
proportionality constant in the directly proportional relationship
S = k × C. (y2 - y1)
k = ————————— =
(x2 - x1)
[3]
Complete this precisely worded conclusion (which incorporates the
constants). 'The speed of hydrogenating 1.00 mole of hexa-1,3,5-triene
in 1 dm³ of ethanol at 23°C, in the absence of ______ and ________, is
directly proportional to the amount (C) of hydrogenase "X" (within the
range ________________); i.e., S = k × C, where k = _________________.'
[3]
Use the equation, and your value of k, to estimate the reaction time if
0.025 mmol of hydrogenase "X" was used. _______________________________
_______________________________________________________________________
[2]
(g) Carefully explain why the speed was slower, k = 0.0035 s-¹ mmol-¹,
when this series of experiments was repeated at 3°C. __________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(h) In related series of experiments, the value of k increased with
increasing temperature upto 55°C - as expected. Suggest why, however,
the value of k was zero at all temperatures above 65°C. _______________
_______________________________________________________________________
[1]
(i) Recombinant DNA technology, popularly known as genetic engineering,
could be used to alter the genes of these anaerobic micro-organisms.
Suggest one desirable improvement in hydrogenase "X". _________________
_______________________________________________________________________
[1]
2. A significant fraction of the human body's cholesterol is used to
biosynthesize bile salts; these have detergent properties similar to
the salts of long-chain carboxylic acids, and so can act as emulsifying
agents for fats and oils. In which organ are bile salts produced? _____
_______________________________________________________________________
[1]
ORGANIC CHEMISTRY: ISOPRENE
Many biologically important molecules, such as abscisic acid (a plant
growth regulator), cholesterol (a component of cell membranes), natural
rubber, oestrogen (a female sex hormone), retinal (a photochemical
pigment in the mammalian eye), and testosterone (a male sex hormone),
are based on a repeating five-carbon unit known as isoprene, "T", which
is biosynthesized from isopentenol, "U".
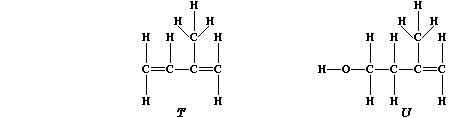
1. Suggest the reason why 'diene' is present in the systematic name of
isoprene (2-methylbuta-1,3-diene). ____________________________________
[1]
2. Manufacturers require far greater amounts of isoprene than can be
obtained from natural sources. Suggest how this synthetic intermediate
is obtained industrially in bulk quantities. __________________________
_______________________________________________________________________
[2]
3. Partial hydrogenation of isoprene gives two liquid alkenes, "V" and
"W": whereas complete hydrogenation gives liquid alkane "X".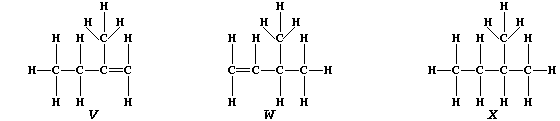
(a) Suggest one physical method, apart from chromatography, which could
distinguish between isomeric alkenes "V" and "W". _____________________
[1]
(b) Describe how you would show that a sample of "X" contained neither
"V" nor "W". __________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
(c) Saturation of diene "T" with the radioactive isotope of hydrogen-3
(tritium), gives labelled alkane "Y".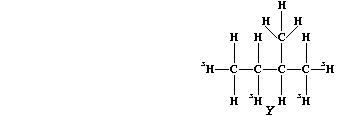
If this isotope of hydrogen (³H) has a half-life of 12.5 years, what
fraction of the labelled alkane "Y" would be present after 37.5 years?
_______________________________________________________________________
[2]
4. In a living organism, alcohol "U" is enzymically dehydrated to
diene "T". Name one catalyst which can speed up a similar dehydration
in the laboratory. ____________________________________________________
[1]
5. Natural rubber, "Z", is poly(isoprene). This hydrocarbon addition
polymer, which occurs as an aqueous suspension in the vascular system
of a number of plants, is usually extracted by 'tapping' rubber trees
(Hevea brasiliensis).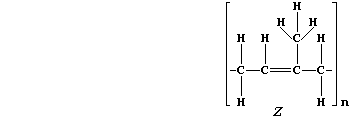
(a) Addition polymers and their corresponding monomers have identical
empirical formula, despite vastly different molecular formulae; e.g.,
poly(tetrafluoroethene), (-C2F4-)n, and tetrafluoroethene, C2F4, both
have the empirical formula CF2. What is the molecular formula of:
Natural rubber? ______________________ Isoprene? ______________________
[2]
(b) The molar mass of a monomeric unit in natural rubber is 68 g mol-¹.
If the molar mass of a sample of natural rubber is 1.02 x 10³ kg mol-¹,
calculate the value of 'n' in the molecular formula. __________________
_______________________________________________________________________
[1]
(c) Ozone, when produced by a complex series of photochemical reactions
involving nitrogen oxides and unburnt hydrocarbons emitted from vehicle
exhausts, is a component of photochemical smog. In sharp contrast to
its rôle in the upper atmosphere, protecting the biosphere from harmful
u.v. radiation, ozone causes the destruction of a diverse range of
synthetic and naturally occurring molecules. For example, the cracks
that appear in vehicle tyres often result from the corrosion of rubber
by ozone; such reactions can be summarized by this first scheme: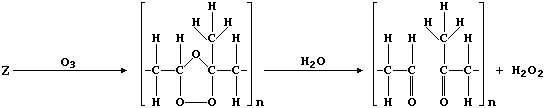
Complete the structural formulae shown in this second scheme, so as to
summarize the effect of ozone on the biosynthetic intermediate "U".
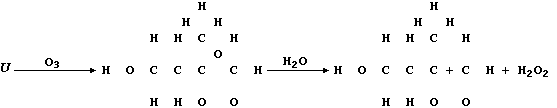
[3]
Suggest one type of naturally occurring molecule, which is involved in
growth or in reproduction, that might also be destroyed (or modified)
by the ozone present in smog. _________________________________________
[1]
(d) The major repositories for the biosphere's gene bank include the
rainforests: so their conservation is essential. The careful management
of rubber trees represents one renewable use of these resources. State
one non-renewable use of rainforests. _________________________________
_______________________________________________________________________
[1]
ORGANIC CHEMISTRY: POLY(CHLOROETHENE)
The characteristic reaction of an alkene is addition across the carbon
double bond, as exemplified by addition polymerization. A particularly
versatile addition polymer is poly(chloroethene), commonly known as PVC
(polyvinylchloride), which is formed from the monomer chloroethene.
1. The monomer chloroethene is manufactured in two steps from ethene:
C2H4 —————————————————————® C2H4Cl2 ——————————————————————® C2H3Cl
(a) Name the product eliminated when 1,2-dichlorethane is converted to
chloroethene. _________________________________________________________
[1]
(b) Suggest one physical method which could determine whether a sample
of chloroethene contained either ethene or 1,2-dichloroethane. ________
_______________________________________________________________________
[1]
(c) Determine the general formula of the homologous series to which
chloroethene belongs. _________________________________________________
[1]
2. The addition polymerization of alkenes occurs under a variety of
reaction conditions. Broadly speaking, high temperatures and pressures
are used to obtain low-density polymers, whereas catalysts are used to
obtain high-density polymers. The equation below summarizes two sets of
typical reaction conditions used to polymerize chloroethene.
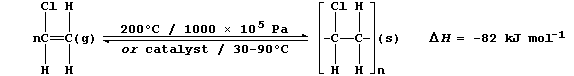
(a) Complete and label the energy level diagram for the polymerization
of chloroethene.
Energy ___
___
_____________ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_____________
_________________
_________________
_________________________________________________
Path of reaction
[4]
(b) Carefully explain one advantage in using high pressures to obtain
(low-density) poly(chloroethene), apart from increasing the speed of
reaction. _____________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) Carefully explain one disadvantage in using higher temperatures to
obtain (high-density) poly(chloroethene), apart from reasons of safety
and cost. _____________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
3. The following description of addition polymerization only refers to
that of chloroethene under conditions of high temperature.
When thousands of molecules of the monomer are heated, the double bonds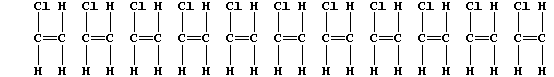
break to produce 'free radicals' (•); these bond inter-molecularly,
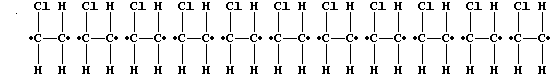
but randomly, to form one polymer molecule:
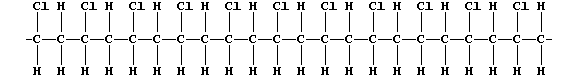
[Polymers with the functional groups arranged randomly along the chain
are known as 'atactic polymers'.]
(a) How many unpaired electrons are there in each free radical? _______
[1]
(b) Suggest what is formed when a free radical bonds intra-molecularly.
_______________________________________________________________________
[1]
4. Aside from conserving non-renewable resources, the recycling of
addition polymers, followed by catalytic cracking and fractional
distillation, should be better for the environment than their disposal:
certainly, present methods cause serious problems. Thus, these plastics
are usually neither bio- nor photo-degradable and, when burnt, produce
gases which are often serious pollutants.
(a) Biodegradable substances are broken down enzymically by which group
of living organisms? __________________________________________________
[1]
(b) What type of energy is involved with photodegradability? __________
[1]
(c) Name one acidic and one toxic gas produced when poly(chloroethene)
is burnt. _____________________________________________________________
[2]
5. The addition reaction of fluorine with chloroethene produces a CFC
(i.e., a chlorofluorocarbon).
(a) Draw the structural formula of this addition product. _____________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) State two environmental effects of CFCs. __________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
ORGANIC CHEMISTRY: UREA
Urea, which is correctly known as carboxamide, has the distinction of
being the first organic compound to have been made in a laboratory
(1828); it is the main nitrogenous excretion product of most mammals.
1. Urea is manufactured from ammonia and carbon dioxide;
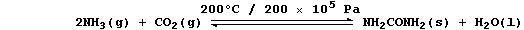
The equation for this condensation reaction can be written as:
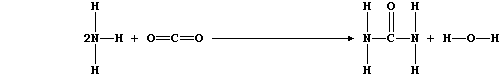
(a) Complete this first Table by inserting the correct numbers of bonds
broken and formed.
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
N-H |
2334 |
N-H |
1156 |
C=O |
1490 |
C=O |
745 |
|
|
C-N |
558 |
|
|
O-H |
926 |
Total = 3824 |
Total = 3785 |
[2]
(b) Calculate the heat energy change (DH) for the above reaction. _____
_______________________________________________________________________
[2]
(c) Carefully explain two reasons why high temperatures are used in the
industrial process. ___________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
2. The nitrogenous fertilizers in commonest use are ammonium salts,
but others are occasionally used; e.g., urea and sodium amide (NaNH2).
(a) Complete this second Table by calculating the molar mass and the
percentage nitrogen of each compound.
Name |
Formula |
M / mol g-¹ |
% Nitrogen |
Ammonium sulfate |
(NH4)2SO4 |
|
|
Ammonium hydrogenphosphate |
(NH4)2HPO4 |
|
|
Ammonium nitrate |
NH4NO3 |
|
|
Urea |
(NH2)2CO |
|
|
[4]
Apart from its higher nitrogen content, suggest one reason why urea is
sometimes preferred to an ammonium salt as a fertilizer. ______________
_______________________________________________________________________
[1]
(b) Various soil micro-organisms secrete urease; this nickel-containing
enzyme catalyzes the conversion of urea to ammonium carbonate in moist
soils. Construct the symbol equation for this reaction. _______________
_______________________________________________________________________
_______________________________________________________________________
[2]
Suggest one pollutant which might inhibit the activity of this enzyme.
_______________________________________________________________________
[1]
3. Urea's principal use is as a monomer in the syntheses of various
condensation polymers that are thermosetting materials (i.e., they do
not soften on heating). One of the many versatile reaction schemes used
is summarized below: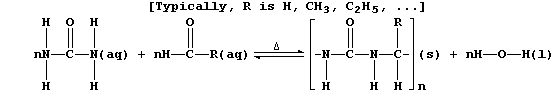
(a) Complete the equation below, which summarizes the conditions used
to prepare the 'parent' polymer (i.e., R = H).
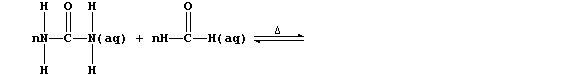
[2]
(b) In contrast to addition polymerization, condensation polymerization
is an endothermic reaction; so, in principle, condensation polymers
should be readily hydrolyzed to their respective monomers. Suggest and
explain one reason why, in practice, synthetic condensation polymers
are usually stable to hydrolysis. _____________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) Naturally occurring condensation polymers include fats, proteins,
carbohydrates, and nucleic acids; their hydrolysis is vitally important
in the transfer and recycling of chemical energy and nutrient ions
between living organisms. For each of the following, name one group of
enzymes involved in their hydrolysis as well as the name of the final
monomeric products.
Carbohydrates _________________________________________________________
[2]
Proteins ______________________________________________________________
[2]
Fats __________________________________________________________________
[3]
(d) Most synthetic condensation polymers, including polyesters such as
Terylene, are thermosetting: but a few, including polyamides such as
Nylon, are thermoplastic (i.e., they soften on heating and harden again
on cooling). By contrast, nearly all synthetic addition polymers are
thermoplastic; the most notable exception is poly(tetrafluoroethene),
popularly known as Teflon, which is thermosetting. Name one addition
polymer, apart from poly(chloroethene), which is a thermoplastic. _____
_______________________________________________________________________
[1]
4. Chemists often use the patterns inherent in the Periodic Table to
justify the syntheses of compounds which should have similar properties
to one already known; e.g., NaCl ('common salt') ——» KCl, NaF, LiI, ...
Suggest the structural formulae of two compounds which might be
expected to show chemical or biological properties similar to urea.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
ORGANIC CHEMISTRY: BIOCIDES
Chemical substances which kill living organisms are known as biocides.
[In Lewis Carroll's story, Through the Looking-Glass (London, 1872),
the Red Queen said: "Now, here, you see, it takes all the running you
can do, to keep in the same place."]
1. Various species of insects are regarded as pests because either
they act as vectors for pathogens (e.g., female Anopheles mosquitoes),
or they destroy crops (e.g., Locusta migratoria). Substances used for
destroying insect pests are known as insecticides, as typified by DDT.
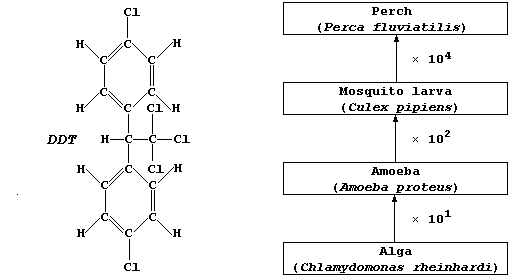
(a) DDT is non-selective, killing beneficial as well as pest species,
and accumulates up the trophic levels. The diagram above represents a
simple food chain in a typical freshwater lake. Estimate the amount of
DDT which could be accumulated by one perch, assuming that each alga
absorbed 1.0 µg and that none of the consumers egest or excrete DDT.
_______________________________________________________________________
[2]
Apart from predators and pollutants, state two factors which limit the
growth of algae. ______________________________________________________
[2]
(b) DDT's over-use has resulted in many short-lived organisms evolving
resistance; e.g., mosquitoes and tsetse flies (Glossina palpalis).
Thus malaria, which is caused by pathogenic protoctistans (Plasmodium),
has been controlled by the use of DDT on adult mosquitoes: but, ever
since DDT was first introduced in 1943, this method's effectiveness has
decreased, inevitably and inexorably, because populations of mosquitoes
resistant to DDT have evolved by the mechanism of natural selection.
Complete the following paragraph using the words from this list:
fittest; increased; inherited; meiosis; variation.
"Structural __________ has occurred in populations of Anopheles gambiae
mosquitoes due to natural mutations, followed by the exchange of genes
via ________ and random fertilization. Some of these mutations have
resulted in some mosquitoes containing dominant alleles which code
for the catabolism of DDT. These individuals have been the ________ in
environments where the agent of selection is DDT, and so more of these
have survived to reproductive maturity. Their offspring have __________
these favourable alleles; and so, within the gene pool of A. gambiae
mosquitoes, the frequency of DDT-resistant alleles has __________."
[5]
State one other method of destroying the vectors of malarial parasites
(Plasmodium), apart from using either pheromones or other insecticides
(e.g., malathion). ____________________________________________________
[1]
2. Bactericides include antiseptics; i.e., chemicals which, when used
at the correct concentration, kill pathogenic but not human cells. The
first antiseptic was 'Carbolic acid' (phenol), introduced by Lister in
1867, and two in household use today are 'TCP' (2,4,6-trichlorophenol)
and 'Dettol' (3,5-dimethyl-4-chlorophenol).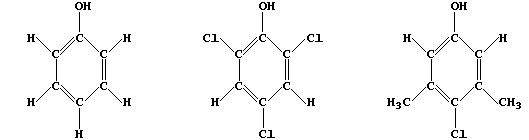
The order of bactericidal activity is: Dettol > TCP > Carbolic acid.
Study the substitution patterns and then, by completing the structural
formulae below, predict two compounds which might result in this order
of bactericidal activity: "U" > Dettol > TCP > "T" > Carbolic acid.
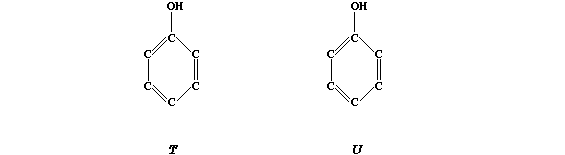
[2]
Apart from being effective in killing pathogenic bacteria, suggest two
desirable characteristics of a bactericide. ___________________________
_______________________________________________________________________
[2]
3. Plants use glucose, produced by photosynthesis, mainly in two ways:
first, as a respiratory substrate; and second, to synthesise all the
other compounds required for growth and reproduction [examples of these
'primary' metabolites include polysaccharides, nucleic acids, proteins,
and oils]. In order to discourage insect predators, many plants have
also evolved an ability to synthesize their own insecticides [examples
of these 'secondary' metabolites include caffeine, mustard, nicotine,
2-hydroxybenzenecarboxylic acid ("V"), and ethanedioic acid ("W")].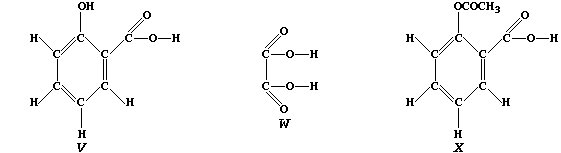
Compound "V", better known as salicylic acid, and structurally related
to aspirin ("X"), is used as an antiseptic and as a food preservative.
Because salicylic acid is only slightly soluble in water, manufacturers
prefer to use the water-soluble sodium salt. So as to summarize one
synthetic method for this salt, complete the following symbol equation:
C6H5OCOOH(aq) + NaHCO3(aq) —————————®
[2]
ORGANIC CHEMISTRY: DETERGENTS
Washing products used to simplify the chore of cleaning clothes include
soaps and detergents. Most soaps are sodium salts of the long-chain
carboxylic acids obtained by hydrolysis of animal fats and plant oils;
and the structural formula of a typical soap, sodium octadecanoate, is:
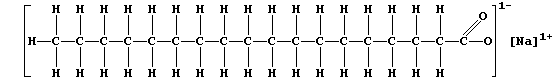
Currently, for most domestic and industrial applications, the preferred
products are the synthetic detergents manufactured from petroleum oil
fractions. This reaction scheme summarizes the synthesis of a typical
detergent, sodium 4-dodecylbenzenesulfonate.
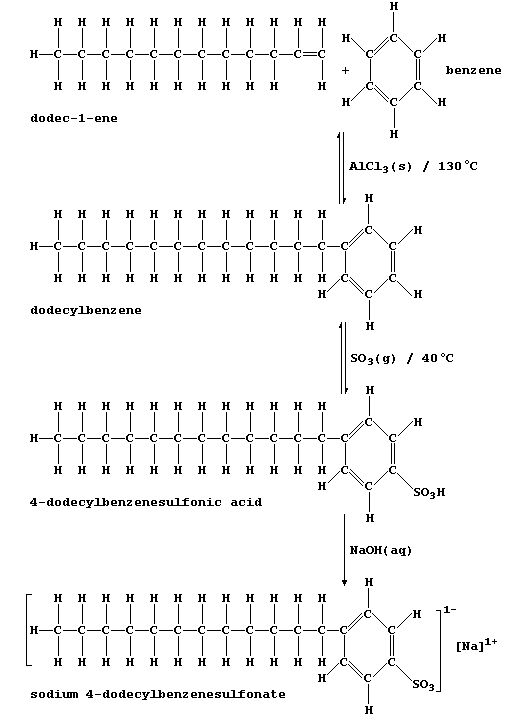
1. Dodec-1-ene and benzene are usually obtained via catalytic cracking
of petroleum oil fractions; e.g., cracking of hexadecane, C16H34, can
produce this straight-chain isomer of dodecene and one other molecule.
(a) Construct the symbol equation for this cracking process. __________
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) Suggest what would be observed if a mixture of alkaline potassium
manganate(VII) and dodec-1-ene was shaken. ____________________________
_______________________________________________________________________
[2]
(c) Details of cracking processes, which often involve co-catalysts
such as Pt-Al2O3, are closely guarded industrial secrets. Nevertheless,
published research indicates that such processes must require careful
control because catalytic isomerization results in branched isomers
being formed. Draw one branched-chain isomer of dodecene. _____________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(d) Detergents made using branched-chain alkenes are not biodegradable.
Explain the meaning of the term 'biodegradable'. ______________________
_______________________________________________________________________
[2]
Suggest one reason why non-biodegradable detergents are considered to
be pollutants. ________________________________________________________
[1]
2. The addition of benzene across the double bond of dodec-1-ene, to
form dodecylbenzene, is analogous to the addition of bromine to ethene.
Predict the name, draw the structural formula, and calculate the molar
mass of the compound formed by the addition of benzene to ethene. _____
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
3. Suggest one advantage in using a low reaction temperature for the
substitution reaction of sulfur trioxide with dodecylbenzene. _________
_______________________________________________________________________
[1]
4. The neutralization of 4-dodecylbenzenesulfonic acid is normally
carried out with sodium hydroxide. Name a less corrosive alkali which
should be suitable for this reaction. _________________________________
[1]
5. The efficacy of detergents is only slightly reduced if hard water
is used, because Group 2 alkylbenzenesulfonates are soluble; i.e., in
contrast to the insoluble Group 2 salts of long-chain carboxylic acids,
precipitates of 'scum' are not produced. Give the formulae of two
substances which cause hardness. ______________________________________
[2]
ORGANIC CHEMISTRY: MALE CONTRACEPTIVES
A research chemist executed studies on the synthesis of chemicals which
might have potential as male contraceptives. The 'lead' compound chosen
was 2,3-diaminopropanoic acid (DAP):
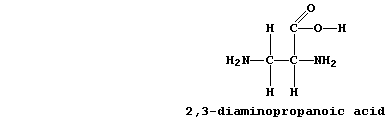
The scientist's reasoning for choosing this particular compound was
complicated, though pivotal to the choice were biological and chemical
similarities of the following four compounds.
Indolyl-3-ethanoic acid, "T", was the first plant growth regulator to
be identified and characterized (1934-1936); its effects are observed
in the familiar tropic activities of plants (e.g., the phototropism
first noted by Charles Darwin in 1880), and it is known to be directly
involved in both protein and RNA synthesis.
Ethene, "U", perhaps surprisingly, is also a plant growth regulator; in
this context, it is used commercially as a method of ripening fruit.
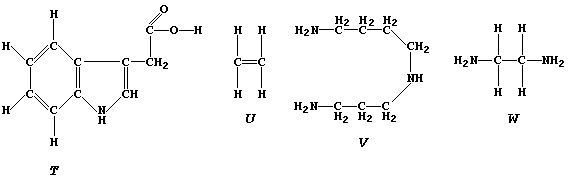
Spermidine, "V", is a natural tri-amine present in the seminal fluid
secreted by mammals.
1,2-Diaminoethane, "W", forms numerous platinum(II) compounds; some of
these show anti-cancer activity and, therefore, most probably affect
the DNA of cancerous cells.
1. Suggest one reason why the exponential increase in human population
is causing concern. ___________________________________________________
[1]
2. Naturally occurring female hormones, which prevent follicle growth
and ovulation, are produced synthetically and incorporated into several
contraceptives. Name two such hormones. _______________________________
[2]
3. State two functional groups which are shared between DAP and one or
more of the compounds "T" to "W". _____________________________________
[2]
4. What do the abbreviations RNA and DNA stand for? __________________
_______________________________________________________________________
[2]
5. Compounds "T" to "W" are directly or indirectly involved with which
two characteristics of living organisms? ______________________________
[2]
6. Plants biosynthesize acid "T" in response to the stimulus of light.
Name two other tropisms and their corresponding stimuli. ______________
_______________________________________________________________________
[4]
7. Ripening fruit requires only a small quantity of ethene, "U"; this
is obtained by the catalytic dehydration of ethanol. Construct the
symbol equation for this reversible reaction. _________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
8. Suggest the reason why spermidine, "V", is called a 'tri-amine'.
_______________________________________________________________________
[1]
9. Noting that certain platinum(II) compounds of "W" show anti-cancer
activity, suggest one other metal ion which might also be effective.
_______________________________________________________________________
[1]
10. The synthetic route chosen by the scientist to 2,3-diaminopropanoic
acid, a known compound, is shown below:
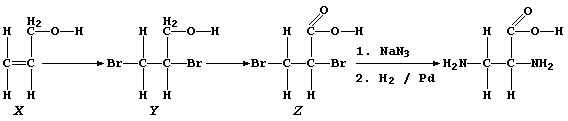
(a) State the colour change observed during the course of the addition
reaction of bromine with alcohol "X". _________________________________
(b) Alcohols "X" and "Y" are liquids at room temperature, with similar
boiling points. What physical technique could be used to separate a
mixture of "X" and "Y"? _______________________________________________
(c) Name a reagent which could oxidize alcohol "Y" to dibromo-acid "Z".
_______________________________________________________________________
(d) Name the gas evolved when a mixture of dibromo-acid "Z" and aqueous
sodium hydrogencarbonate was warmed. __________________________________
(e) The conversion of dibromo-acid "Z" to DAP involves a substitution
reaction and a reduction (step 2). Suggest the purpose of the palladium
in this second step. __________________________________________________
(f) Suggest one physical test used to confirm that DAP, a crystalline
substance, had been obtained. _________________________________________
[6]
(g) The scientist considered an alternative to the synthetic route to
DAP; specifically, a fermentation process using propene as the starting
material. Suggest two potential advantages of this process. ___________
_______________________________________________________________________
[2]
(h) Tests on laboratory rats showed that DAP was partially successful;
thus, rats injected with DAP were less fertile than the controls (i.e.,
those used to isolate the independent variable; these were not injected
with DAP). Accordingly, more compounds were synthesized - including two
isomers obtained by the hydration of compound "X". Draw the structural
formula of each isomer. _______________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
(i) This research was shelved as soon as some potentially devastating
news began to circulate in the early 1980s. Suggest one disadvantage in
using chemical contraceptives. ________________________________________
_______________________________________________________________________
[1]
_______________________________________________________________________
_______________________________________________________________________
Comedy-Dramas for Year 10-12 Students in British Orthography :
[No. 1] [No. 2] [No. 3] [No. 4] [No. 5] [No. 6] [No. 7] [No. 8] [No. 9]
[Dr. Roger Peters : rpeters@wissensdrang.com ; Aufbau1 ; Home Page]