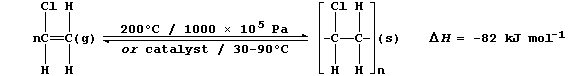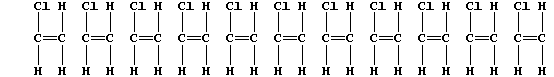ORGANIC CHEMISTRY: POLY(CHLOROETHENE)
The characteristic reaction of an alkene is addition across the carbon
double bond, as exemplified by addition polymerization. A particularly
versatile addition polymer is poly(chloroethene), commonly known as PVC
(polyvinylchloride), which is formed from the monomer chloroethene.
1. The monomer chloroethene is manufactured in two steps from ethene:
C2H4 —————————————————————® C2H4Cl2 ——————————————————————® C2H3Cl
(a) Name the product eliminated when 1,2-dichlorethane is converted to
chloroethene. _________________________________________________________
[1]
(b) Suggest one physical method which could determine whether a sample
of chloroethene contained either ethene or 1,2-dichloroethane. ________
_______________________________________________________________________
[1]
(c) Determine the general formula of the homologous series to which
chloroethene belongs. _________________________________________________
[1]
2. The addition polymerization of alkenes occurs under a variety of
reaction conditions. Broadly speaking, high temperatures and pressures
are used to obtain low-density polymers, whereas catalysts are used to
obtain high-density polymers. The equation below summarizes two sets of
typical reaction conditions used to polymerize chloroethene.
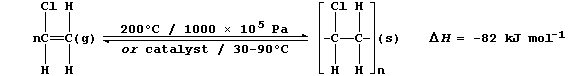
(a) Complete and label the energy level diagram for the polymerization
of chloroethene.
Energy ___
___
_____________ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_____________
_________________
_________________
_________________________________________________
Path of reaction
[4]
(b) Carefully explain one advantage in using high pressures to obtain
(low-density) poly(chloroethene), apart from increasing the speed of
reaction. _____________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) Carefully explain one disadvantage in using higher temperatures to
obtain (high-density) poly(chloroethene), apart from reasons of safety
and cost. _____________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
3. The following description of addition polymerization only refers to
that of chloroethene under conditions of high temperature.
When thousands of molecules of the monomer are heated, the double bonds
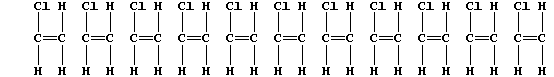
break to produce 'free radicals' (•); these bond inter-molecularly,
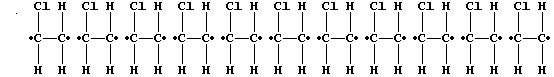
but randomly, to form one polymer molecule:
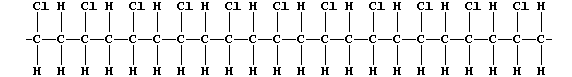
[Polymers with the functional groups arranged randomly along the chain
are known as 'atactic polymers'.]
(a) How many unpaired electrons are there in each free radical? _______
[1]
(b) Suggest what is formed when a free radical bonds intra-molecularly.
_______________________________________________________________________
[1]
4. Aside from conserving non-renewable resources, the recycling of
addition polymers, followed by catalytic cracking and fractional
distillation, should be better for the environment than their disposal:
certainly, present methods cause serious problems. Thus, these plastics
are usually neither bio- nor photo-degradable and, when burnt, produce
gases which are often serious pollutants.
(a) Biodegradable substances are broken down enzymically by which group
of living organisms? __________________________________________________
[1]
(b) What type of energy is involved with photodegradability? __________
[1]
(c) Name one acidic and one toxic gas produced when poly(chloroethene)
is burnt. _____________________________________________________________
[2]
5. The addition reaction of fluorine with chloroethene produces a CFC
(i.e., a chlorofluorocarbon).
(a) Draw the structural formula of this addition product. _____________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) State two environmental effects of CFCs. __________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Dr. R. Peters Next Contents' List