METALS: STRONTIUM
Strontium, a relatively rare element in the Earth's crust (0.04%),
occurs mainly as the sulfate or carbonate (e.g., in the ores celestite
and strontianite, respectively). Strontium compounds have neither any
large-scale industrial uses nor any known biological rôles. However,
partly because of the observed and predicted similarities between
calcium and strontium ions, and partly because calcium ions have been
shown to be essential to all living organisms, strontium ions could be
expected to affect diverse biochemical processes.
[.. K > Ba > Sr > Ca > Na > Mg > Al > Zn > Fe > Pb > (H) > Cu > Hg ..]
1. Based on strontium's position in the reactivity series, suggest how
the metal could be extracted from its chloride. _______________________
_______________________________________________________________________
[2]
2. This first Table shows the temperatures necessary to induce, and
the heat energy changes accompanying, the thermal decomposition of four
Group 2 carbonates.
|
BaCO3 |
SrCO3 |
CaCO3 |
MgCO3 |
Decomposition temperature / °C |
1360 |
1290 |
900 |
400 |
Heat energy change (DH) / kJ mol-¹ |
269 |
235 |
178 |
101 |
A radiochemist used the carbon-14 isotope to prepare a small amount of
radioactive labelled strontium carbonate, Sr14CO3. Using the apparatus
shown, a sample of this labelled compound was heated; the evolved gas
passed into limewater, and the precipitate collected by filtration.
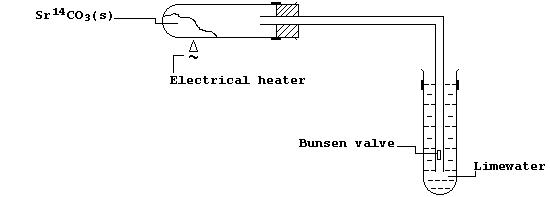
(a) Ensuring you indicate the radioactive-labelled carbon, complete and
label the energy level diagram for the endothermic decomposition of
strontium carbonate.
Energy ___
___________________
___________________
____________ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
____________
______________________________________________
Path of reaction
[5]
(b) Construct the symbol equation for the reaction of the evolved gas
with limewater. _______________________________________________________
_______________________________________________________________________
[2]
3. Strontium carbonate could be reasonably viewed as an 'analogue' of
calcium carbonate, which is a major component of the exoskeletons of
various protoctistans, sponges, corals, crustaceans, and molluscs.
Temporary hard water results from the slow reaction of rainwater with
naturally occurring calcium carbonate; its 'strontium equivalent' is:
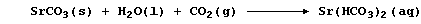
Complete this second Table to show further strontium analogues.
|
Calcium compound |
Strontium analogue |
Mammalian endoskeleton |
Ca5(PO4)3OH(s) |
Sr5(PO4)3OH(s) |
Crustacean exoskeleton |
CaCO3(s) |
SrCO3(s) |
Permanent hard water |
|
|
Temporary hard water |
|
|
'Scale' or 'fur' |
|
|
Limewater |
|
|
[2]
Construct the symbol equation for each of these strontium equivalents.
Softening permanent hard water ________________________________________
_______________________________________________________________________
Softening temporary hard water ________________________________________
_______________________________________________________________________
Removing scale with aqueous methanoic acid, HCOOH(aq) _________________
_______________________________________________________________________
[6]
4. Aqueous strontium hydroxide contains the ions Sr2+(aq), OH1-(aq),
and H1+(aq). When this solution is electrolyzed, using carbon-graphite
electrodes, these reactions occur: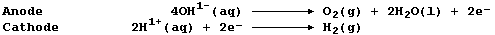
Explain, using a symbol equation, each of the following observations
made during such an electrolysis experiment.
The mass of the anode decreased. ______________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
The solution turned milky during the initial stages of electrolysis. __
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
The milky suspension turned clear if electrolysis was continued. ______
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
5. One radioactive isotope of strontium, Sr-90, has a half-life of 28
years and is a b-emitter (i.e., it decays spontaneously, emitting
radiation in the form of high speed electrons). This isotope, one of
the most dangerous waste products of the nuclear industry, is a fission
product in nuclear explosions and in reactors of nuclear power plants.
Suggest what might happen to any compounds of strontium-90 ingested by
a mammal. _____________________________________________________________
_______________________________________________________________________
[2]
If 0.16 g of strontium-90 was absorbed into the exoskeleton of a coral
(e.g., Corallum rubrum), how much would remain after 84 years? ________
_______________________________________________________________________
[2]
Name one process in autotrophs or saprotrophs that might be affected by
strontium-90. _________________________________________________________
[1]
Dr. R. Peters Next Contents' List