METALS: EXTRACTION of ALUMINIUM
Aluminium, the commonest metal in the Earth's crust (8.3%), is usually
found as the oxide (e.g., in the ore bauxite). Typical of main group
metals, aluminium has a fairly low melting point (660°C), a low density
(2.7 g cm-³), and forms (usually) white or colourless compounds in only
one oxidation state (III).
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. Aluminium is extracted by the electrolytic reduction of purified
molten bauxite; the ionic equations for the reactions occurring at the
electrodes are:
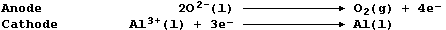
(a) Label this diagram of an industrial electrolytic cell with:
Graphite anode; Graphite cathode; Insulator; Molten aluminium; Molten
electrolyte; and, Solid crust of electrolyte.
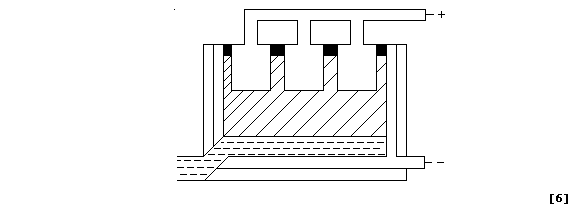
(b) To lower the melting point of aluminium oxide from about 1500°C to
1000°C, and so reduce energy costs, cryolite (Na3AlF6) is added to the
bauxite. Explain why (pure) aluminium oxide has a high melting point.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
(c) Name one toxic gas that might arise from the thermal decomposition
of cryolite. __________________________________________________________
[1]
(d) The anode needs to be replaced periodically because the carbon is
completely oxidized by the evolving dioxygen. Name the toxic pollutant
formed by partial oxidation of the carbon anode. ______________________
[1]
[Q = n × z × F and Q = I × t, where: Q, measured in coulombs (C), is
the quantity of electricity; n is the number of moles of substance
evolved at the electrode; z is the charge on the ion; F is a constant,
with a value of 96500 C mol-¹; I, measured in amps (A), is the current;
and t, measured in seconds (s), is the time.]
2. In industry, a typical electrolytic cell operates continuously at a
current of 4000 kA. Determine the mass (m) of aluminium that forms at
the cathode every day - as follows.
Calculate the quantity of electricity (Q) used every 24 hours. ________
_______________________________________________________________________
Calculate the number of moles (n) of aluminium formed at the cathode.
_______________________________________________________________________
And finally, calculate the mass (m) of aluminium formed at the cathode.
_______________________________________________________________________
[6]
3. Electrolytic reduction, which is often used for the extraction of
the most reactive metals, requires expensive electrical energy: by
contrast, chemical reduction with carbon uses a relatively cheap source
of chemical energy. Which method of generating electrical power is the
cheapest and most environmentally acceptable? _________________________
[1]
297 kJ of energy are required to produce 1 mole of aluminium metal by
electrolysis, whereas only 26 kJ of energy are required to recycle
1 mole. Calculate the percentage energy saved by recycling aluminium.
_______________________________________________________________________
[2]
4. Aluminium's high natural abundance, high resistance to corrosion,
high thermal conductivity, low density, and low electrical resistance,
has resulted in its widespread use (e.g., in window frames, cooking
utensils, food and drink cans, aircraft, and electrical power lines).
The metal surface is invariably covered by a very thin layer of oxide,
because pure aluminium is rapidly oxidized by atmospheric oxygen;
This layer is non-porous and protects the metal from further corrosion,
providing it is not exposed to aqueous chloride ions.
(a) Paralleling aluminium's increased use in cooking utensils and in
food containers, there has been an increase in the number of people who
suffer from Alzheimer's disease. This parallel may indeed be a most
unfortunate coincidence, rather than a correlation, but one partial
explanation could be as follows. Food is often preserved in brine and
cooked with salt; so, should the protective layer of aluminium oxide in
the containers be removed by aqueous chloride ions, soluble aluminium
ions could be formed, ingested, and then absorbed into the bloodstream.
Construct an explanation, complete with two symbol equations, for the
assertion that it could be hazardous to use aluminium foil when cooking
salted meat in the presence of steam. _________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
(b) When the thin layer of oxide is made thicker, by anodizing, then
it can easily absorb variously coloured dyes. Shown below is a diagram
of a typical electrolytic cell used to anodize aluminium objects.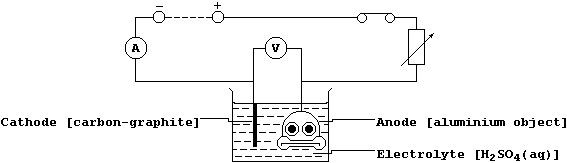
Oxygen gas is evolved at the anode from the electrolysis of an aqueous
solution containing H1+(aq), OH1-(aq), and SO42-(aq) ions; the ionic
equations for the reactions occurring at the electrodes are:

The aluminium object immediately combines with this evolved oxygen gas
to form more aluminium oxide on its surface. Suggest two reasons why
aluminium is anodized. ________________________________________________
_______________________________________________________________________
[2]
Dr. R. Peters Next Contents' List

