SELECTED PRINCIPLES: INTRODUCTION - STRUCTURE and BONDING (PREAMBLE)
The intimately related topics of atomic structure and chemical bonding
are universally regarded as the 'heart of Chemistry'; not surprisingly,
therefore, one's knowledge of these topics and this science increase in
parallel. Rather encouragingly, a student and a mature scientist share
similar mental images of both atomic and molecular structure, as can be
illustrated using a common point of reference; thus, ...
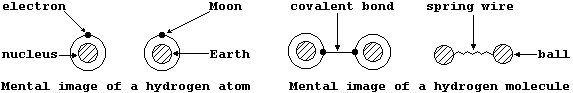
Although neither of these mental images has the slightest relationship
either to physical reality or to their respective analogies, they do
provide a quite convenient starting point from which ever-increasingly
detailed 'pictures' can be drawn ...
The following flow diagram shows a possible pathway to a comprehensive
understanding of either of these two topics: Mental image (student and
mature scientist) ———® Sketch 1 (student) ———® Sketch 2 ——® Finished
drawing ———® Water colour ———® Oil painting (? ... mature scientist).
Ideally, a student needs to acquire sufficient knowledge, on the one
hand, to be able to draw Sketch 1, and on the other, which will allow
Sketch 2 to be drawn (at a later date) using a rubber and a pencil -
without the need to discard Sketch 1 for a new piece of paper.
For decades, the received wisdom has been that Sketch 1 should be drawn
using theories formulated in the 1910s. In particular, these involve
viewing the electron purely as a particle; and, in terms of presenting
an introduction which is easier to understand, this restrictive view
has undoubted advantages. Nevertheless, the properties of an electron
are consistent with its dual behaviour as a particle and a wave. The
evidence for, and acceptance of, this duality forms the basis of Sketch
2 (and with it comes a spectacular increase in one's understanding of
Chemistry).
In this introduction, apart from one mischievous exception, no attempt
has been made to draw Sketch 1 using contemporary theories. However, a
robust framework is presented which is consistent with two unifying
concepts that have remained unchanged with the advances in theoretical
knowledge: first, electrons occupy either atomic or molecular energy
levels; and second, chemical bonding occurs because the resulting
molecule or compound has a lower energy than its constituent atoms.
Furthermore, because the limitations of Sketch 1 are stated, and the
necessary simplifications are kept to a minimum, it should be possible
to draw Sketch 2 using the same piece of paper.
Energy Levels ... Precepts
Each diagram in Figures (a-h) show an infinite number of steps, each
1.0 m in height, and a 1.0 kg ball which is either occupying a step or
being transferred from one step to another; each step may be correctly
and usefully viewed as an energy level.
Figures (a-h)
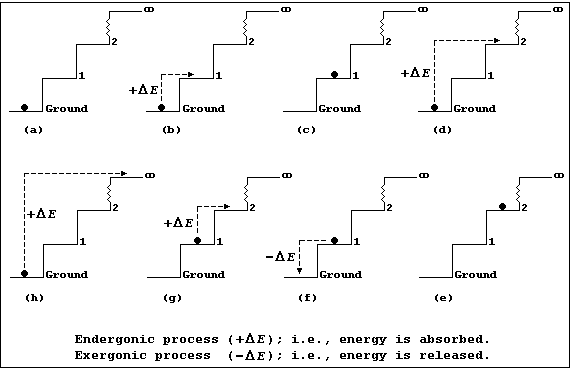
Figure (a) shows a 1 kg ball occupying the ground step; all the other
steps are unoccupied. The energy (E) of the 1.0 kg ball is 0 kJ (i.e.,
E = m × g × h = 1.0 × 10 × 0.0 = 0 kJ).
Figures (b) and (d) show, respectively, the ball being transferred from
the ground to the 1st step and from the ground to the 2nd step; each
process is endergonic (+DE); i.e., energy is absorbed (for example, by
transducing chemical or mechanical energy to potential energy, via
kinetic energy).
Figures (c) and (e) show, respectively, the ball now occupying the 1st
and 2nd steps; it has acquired 10 kJ of potential energy on the 1st
step [Figure (c)] and 20 kJ on the 2nd step [Figure (e)].
Figure (f) shows the ball being transferred back from the 1st to the
ground step: this process is exergonic (-DE); i.e., energy is released
(which, typically, may be as heat and sound energy transduced from
potential energy, via kinetic energy).
Figure (g) shows the ball being transferred from the 1st to the 2nd
step; once transferred it will have acquired another 10 kJ of potential
energy. And, obviously, each similar transfer from a lower to a higher
step (or energy level) will result in the ball acquiring the additional
potential energy.
Figure (h) shows the ball being transferred from the ground step to the
(idealized) infinite step, where the Earth's gravitational force will
be zero; and, here, the ball will no longer be attracted to the Earth.
So, with mischief aforethought, one can say that the hugely endergonic
process of transferring the ball from the ground step to infinity, in
discrete steps, will result in the ball becoming 'ionized'.
And finally, these precepts hold true: firstly, even when the steps are
of non-uniform height (an interesting architectural phenomenon), though
the numerical values would be different; and secondly, in both free and
bonded atoms, where energy levels of non-uniform height tend to be the
rule rather than the exception.
People who are 'positively bursting with the joys of spring' have been
known to express a firmly held opinion: namely, that a swim in ice-cold
water is refreshing after a hot sauna. Presented below is the chemical
equivalent of such a swim: namely, definitions of the commonest terms
used in structure and bonding (a few of these have been simplified).
Matter is anything that occupies space and has mass.
A pure substance is a form of matter that has both definite composition
and distinct properties.
An element is a pure substance which cannot be broken down into simpler
substances by chemical means.
An atom is the smallest particle of an element that can exist and still
retain the ordinary chemical properties of that element.
A proton is a sub-atomic particle, within the nucleus of an atom, which
has unit mass and unit positive charge.
The atomic number (Z) of an element is the number of protons each atom
of that element has in its nucleus; e.g., Z = 17 for chlorine (17Cl).
A neutron is a sub-atomic particle, within the nucleus of an atom,
which has unit mass and zero charge.
The mass number (A) of an atom is the number of protons and neutrons it
has in its nucleus; e.g., A = 35 for a chlorine atom which contains 18
neutrons (35Cl), and A = 37 for one which contains 20 neutrons (37Cl).
Isotopes are atoms of the same element that contain different numbers
of neutrons; e.g., 63Cu and 65Cu are two isotopes of copper.
An electron is a sub-atomic particle, outside the nucleus of an atom,
which has nearly zero mass and unit negative charge.
An ion is an atom (or group of atoms) which has either gained or lost
electrons; e.g., a chloride anion, 17Cl1-, contains 18 electrons (i.e.,
one more than neutral atom, 17Cl0), and a copper(II) cation, 29Cu2+,
contains 27 electrons (i.e., two less than the neutral atom, 29Cu0).
A compound is a pure substance which contains two or more different
elements chemically bonded together in stoichiometric proportions.
A molecule is the smallest part of an element or of a covalently bonded
compound that can exist independently and still retain the ordinary
chemical properties of that element or compound.
A mixture consists of two or more substances which are not chemically
bonded together.
A localized covalent bond describes the mutual electrostatic attraction
of two adjacent nuclei for a shared pair of electrons which occupy the
same molecular energy level.
A delocalized covalent bond describes the electrostatic attraction of
more than two nuclei for a shared pair of electrons which occupy the
same molecular energy level.
An ionic bond describes the electrostatic attraction of two oppositely
charged ions in a crystalline lattice.
Dr. R. Peters Next Contents' List

