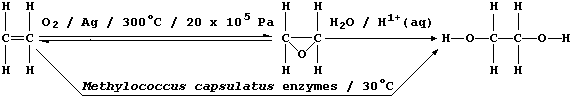ORGANIC CHEMISTRY: BIOTECHNOLOGY
Currently, in developed countries, most energy sources and vast numbers
of chemicals and manufactured products are derived from gas, oil, and
coal. To conserve these non-renewable fossil fuels, as well as to
reduce pollution, alternative sources of energy need to be harnessed.
Encouragingly, all of the major chemical companies have begun to apply
biotechnology; i.e., the industrial use of genes from micro-organisms
to synthesize products considered useful to Man. Chemicals which have
been made using biotechnology include benzylpenicillin (an antibiotic),
ethanol, ethane-1,2-diol, insulin (a hormone), poly(hydroxybutyrate)
(a biodegradable thermoplastic), rennin (an enzyme), and single-cell
protein (SCP; a food).
1. Ethanol is burnt in vehicle engines, instead of petrol, in several
developing countries, partly to reduce their dependence on (imported)
oil and partly as a conservation measure. This ethanol is obtained via
the fermentation of sucrose solutions with yeast; i.e.,
Saccharomyces enzymes / 35°C
C12H22O11(aq) + H2O(l) —————————————————————® 4C2H5OH(aq) + 4CO2(g) -DE
(a) Sugar cane (Saccharum officinarum) and sugar beet (Beta vulgaris)
are the main natural sources of sucrose; the structural formula of this
disaccharide is:
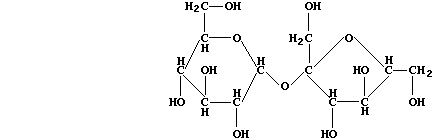
In order to hydrolyze sucrose, Saccharomyces species biosynthesize the
enzyme saccharase; this biological catalyst is roughly 10¹² times more
effective than the typical acid catalysts used in laboratories. State
the molecular formula of glucose and fructose, which are the (isomeric)
monosaccharides produced when this disaccharide is hydrolyzed. ________
[1]
(b) In anaerobic cellular respiration, with glucose as the respiratory
substrate, the term 'alcohol fermentation' strictly refers to the final
pair of eleven reactions (each catalyzed by a specific enzyme); though,
in practice, the term 'fermentation' is being used increasingly to
describe a wide variety of biotechnological processes. Explain why the
speed of fermentation is usually slower at 15°C than at 35°C. _________
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) The mixture at the end of fermentation is first filtered, to remove
suspended particles (in particular, the increased mass of yeast), and
then fractionally distilled, to separate the ethanol from the water.
State two major costs of either of these physical processes. __________
_______________________________________________________________________
[2]
(d) Construct the symbol equation for the combustion of ethanol. ______
_______________________________________________________________________
[2]
(e) Ethanol is usually manufactured in developed countries by catalytic
hydration of ethene. Construct the symbol equation for this reversible
reaction. _____________________________________________________________
_______________________________________________________________________
[2]
2. Ethane-1,2-diol, commonly known as ethylene glycol, is a liquid at
room temperature; it is used as a solvent, as a starting material for
a range of chemicals (e.g., polyesters and polyurethanes), and as an
'antifreeze' (e.g., in car radiators). In industry, this compound used
to be synthesized exclusively by a process which requires dioxygen
(from the air), a silver catalyst, and a high reaction temperature and
pressure: relatively recently, however, a fermentation process using
Methylococcus capsulatus bacteria has been developed.
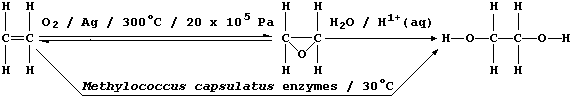
(a) State the empirical formula of ethane-1,2-diol. ___________________
Determine the general formula of the homologous series to which ethane-
1,2-diol belongs. _____________________________________________________
[2]
(b) In the industrial synthetic process, suggest one disadvantage in
using a silver catalyst. ______________________________________________
[1]
(c) Suggest one reason why higher temperatures are not used in the
fermentation process, apart from minimizing costs. ____________________
_______________________________________________________________________
[1]
(d) Suggest one disadvantage in using ethene as the starting material.
_______________________________________________________________________
[1]
3. Shown below are the structural formulae of water, ethanol, ethane-
1,2-diol, and propane-1,2,3-triol (glycerol). Rather atypically for
organic compounds, these three alcohols are miscible with water in all
proportions; their solubility is partially attributable to 'hydrogen
bonding' [i.e., the weak electrostatic attraction between the nucleus
of a bonded hydrogen atom and a 'lone-pair' of electrons of a bonded
non-hydrogen atom (typically, nitrogen or oxygen)].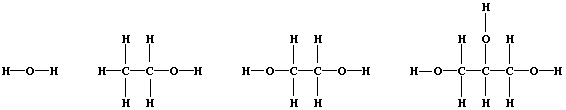
(a) Glycerol's main rôle in living organisms is as an intermediate in
the biosynthesis of fats and oils. However, certain organisms living in
cold habitats also incorporate glycerol into their cellular cytoplasm
to act as an antifreeze. Name one physical process which is slowed down
when biological cells are frozen. _____________________________________
[1]
(b) Glycerol, which is used extensively in the manufacture of solvents,
explosives, and polymers, is obtained by the hydrolysis of fats and
oils using either alkalis or lipases. Suggest one advantage in using
enzymes to effect these hydrolyses. ___________________________________
[1]
(c) Providing either a suitable micro-organism can be isolated or the
genes coding for the biosynthesis of glycerol can be identified, what
might be a suitable starting material to obtain this triol by
fermentation? _________________________________________________________
[1]
Dr. R. Peters Next Contents' List