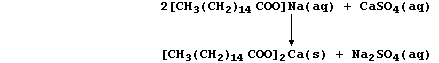METALS: CALCIUM & MAGNESIUM
Calcium, (m.pt. = 839°C; r = 1.54 g cm-³), is the third and magnesium,
(m.pt. = 649°C; r = 1.74 g cm-³), is the fifth most abundant metal in
the Earth's crust (4.2% and 2.3%, respectively); as the Table below
shows, they occur in a variety of minerals and ores. In common with
several other Group 1 and 2 elements, both metals are often extracted
by the electrolytic reduction of their molten chlorides.
Name of mineral or ore |
Composition or principal component |
Gypsum |
CaSO4.2H2O |
Limestone, calcite, chalk, marble |
CaCO3 |
Dolomite |
CaCO3.MgCO3 |
Magnesite |
MgCO3 |
Epsomite |
MgSO4.7H2O |
Despite their relative abundances, neither metal is used extensively in
industry, although both are used in alloys and for chemical reductions:
nevertheless, in the long term, magnesium may replace aluminium as the
low-density structural metal of choice because the supply available in
seawater is virtually unlimited. By way of contrast, both calcium and
magnesium ions have been shown to be essential to all living organisms,
and both affect the 'hardness' of water (one aspect of water quality
considered to be important in developed countries).
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. The presence of nitrate ions (leached from farms), or toxic heavy
metal ions (leached from mines), or calcium ions (which contribute to
hardness), is rarely an important consideration of water quality in
developing countries - where survival frequently depends on obtaining
enough water which is sufficiently pathogen-free. When favourable
conditions prevail, water is purified by boiling (a process which kills
pathogens by irreversibly denaturing their enzymes). Name one pathogen
which is often present in water contaminated with untreated sewage.
_______________________________________________________________________
[1]
2. This diagram shows a simplified plan of a typical water-treatment
works in a developed country.
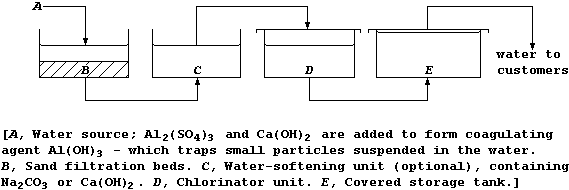
(a) Name one urban water source. ______________________________________
[1]
(b) Suggest the purpose of the:
Sand filtration beds __________________________________________________
Chlorinator unit ______________________________________________________
[2]
(c) Each storage tank must be covered. Suggest one biological reason
why sunlight should be prevented from reaching the water. _____________
_______________________________________________________________________
[1]
3. 'Hard water', which is water that does not readily form a lather
with soap, is caused by the presence of any dissolved Group 2 ions -
though the focus here is on those of calcium [i.e., aqueous Ca(II)] ...
Unpolluted rainwater is effectively a dilute solution of carbonic acid,
H2CO3(aq), and 'temporary' hard water results from the slow reaction of
rainwater with minerals or ores containing calcium carbonate:
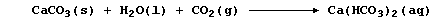
Water containing soluble calcium hydrogencarbonate is considered to be
temporarily hard because the hydrogencarbonate is thermally unstable;
thus, when it is thermally decomposed, soluble calcium ions are removed
from solution as a precipitate - commonly known as 'scale' or 'fur':
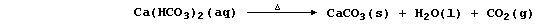
'Permanent' hard water results from the slow (and slight) dissolution
of the calcium sulfate present in minerals and ores:
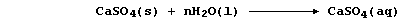
Water containing dissolved calcium sulfate is considered to be
permanently hard because this type of hardness cannot be removed by
boiling; indeed, removal of the soluble calcium ions requires the use
of an 'ion exchanger' or a 'water-softener' (e.g., sodium carbonate):
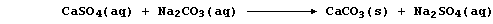
(a) Construct a single symbol equation, involving naturally occurring
magnesite, for the formation and removal of temporary hard water. _____
_______________________________________________________________________
[2]
(b) The structural formula of a typical soap, sodium hexadecanoate, is: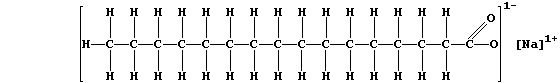
The anions of this soap, better known as sodium palmitate, react with
the calcium ions present in permanent hard water to form a precipitate
of 'scum':
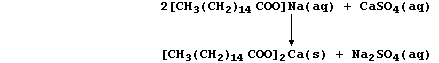
Complete a similar equation for the reaction of these anions with the
calcium ions present in temporary hard water:
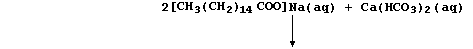
_______________________________________________________________________
[2]
(c) In hard water areas, scale precipitated in pipes and in tanks can
be removed safely by neutralization with an aqueous solution of a weak
acid [e.g., HCOOH(aq)]. Suggest and explain, using a symbol equation,
why farmers usually prefer to remove scale with dilute nitric acid. ___
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
4. State two biologically important rôles of calcium ions, apart from
the formation of exo- and endo-skeletons. _____________________________
_______________________________________________________________________
[2]
5. Name and state the function of one biological molecule that
contains magnesium ions. ______________________________________________
_______________________________________________________________________
[2]
Dr. R. Peters Next Contents' List