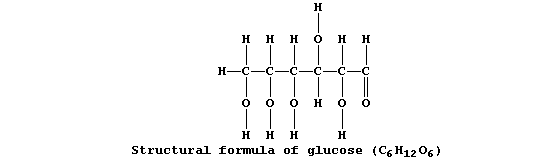REFERENCE SECTION: CHEMICAL EQUATIONS (2)
There are two pivotal moments in the development of a toddler: first,
when he or she realizes that 'Postie' probably does not hop on a sleigh
to the North Pole; and second, when he or she decides to collude with
adults in perpetuating the polite fiction of Father Christmas. Perish
the thought that examples of such fiction occur in Chemistry, but note
carefully the following sentence (J. Barrett, Understanding Inorganic
Chemistry, Ellis Horwood, Chichester, 1991): "At the school level, some
earlier theories are put forward as present-day explanations."
Perdre la grâce (ou Tomber en disgrâce)
Introductory courses invariably include a suite of experiments designed
to illuminate the important differences between elements, mixtures, and
compounds. By long-established tradition, the commonest suite involves
the following substances: the elements iron and sulfur; a mixture of
iron and sulfur; and the 'compound' iron(II) sulfide ...
A dark-grey solid is formed when a mixture of the above two elements is
gently heated; hitherto, this (fairly vigorous exothermic) process has
been summarized by the following equation.

This dark-grey solid, usually designated as the stoichiometric compound
iron(II) sulfide (FeS), has different chemical properties to both the
elements and the mixture; e.g., dilute hydrochloric acid reacts slowly
with iron to give the gas dihydrogen,
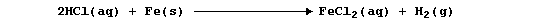
whereas the same acid reacts rapidly with the dark-grey solid to give
the (toxic) gas hydrogen sulfide,
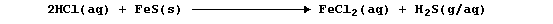
[Incidentally, this latter reaction is a splendid counterbalance to the
oft-stated paradigm 'acid + base gives salt + water only'.]
Chemists had known for decades that the designation of this dark-grey
solid as the stoichiometric compound iron(II) sulfide was not correct:
but, only in the 1980s did researchers show that substance obtained was
a mixture of phases (often called, rather loosely, 'non-stoichiometric'
compounds). No attempt is made here either to summarize this research *
or to explain its importance, simply because the science of phases is
so frightfully complicated: however, do note that a line is placed over
the nominal formula to indicate that a substance is non-stoichiometric,
and that chemical equations are modified; e.g.,

The 'fall from grace' of iron(II) sulfide as the archetypal compound
should, eventually, result in the removal of the suite mentioned above.
Surprisingly, perhaps, there are very few alternatives which fulfil the
two criteria of illumination and safety; of these, and by far the most
attractive, is a suite involving the following substances: the elements
zinc and iodine; a mixture of zinc and iodine; and the compounds water
and zinc iodide. To summarize the results of this suite might well be
regarded as profligate, but two cryptic 'cluettes' are surely in order.
The synthesis of aqueous zinc iodide is an exergonic process; i.e.,
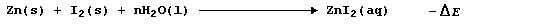
By contrast, but with a delightful feeling of logic, the electrolytic
decomposition of aqueous zinc iodide is an endergonic process; i.e.,
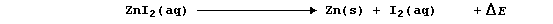
* See: J. K. Burdett and E. L. Miller, J. Am. Chem. Soc., 1987, 4081
(for a specific reference); F. A. Cotton and G. Wilkinson, Advanced
Inorganic Chemistry, Wiley, New York, 1988 (for a concise summary).
Cela sert à faire bouillir la marmite
Collectively, living organisms use a variety of respiratory substrates
as sources of chemical energy [including ethanol, glucose, and iron(II)
sulfide]; of these, the most extensively used is glucose:
Equation 1, or its word equivalent, has been used in examination papers
and textbooks to summarize one fermentation process involving glucose.
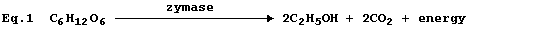
However, as written, equation 1 has little or no merit ...
First, and perhaps most importantly, equation 1 violates the Principle
of the Conservation of Energy - which states 'energy is neither created
nor destroyed, but can be transduced from one form to another'. All
reactions involve an energy change (DE): but energy is never produced.
Second, equation 1 is often - but, admittedly, not always - stated to
be a summary of the fermentation reaction: but this particular example
of fermentation summarizes a biological process that involves at least
eleven separate reactions.
Third, the inclusion of the word zymase is correct: but potentially
misleading. Thus, by analogy with amylase and catalase, amongst others,
one might reasonably assume that zymase is a single enzyme: whereas
zymase is a biological mixture, extracted from yeast (Saccharomyces),
that contains at least eleven enzymes.
And fourth, in the absence of state symbols, reactants are assumed to
be in their normal states at room temperature. In the solid state, the
rate of reaction between glucose and zymase would be immeasurably slow,
because there would be remarkably few collisions between the particles.
The above (rather brutal) deconstruction would be of precious little
value unless an acceptable alternative is available; happily, Equation
2 provides a most satisfactory summary of this fermentation process.
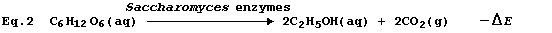
This fermentation process is but one example of respiration, whose
purpose in a living organism is to release energy in the usable form of
adenosine triphosphate (ATP). * Accordingly, the use of Equation 3 to
summarize this fermentation would be misleading, because it would imply
that the purpose of respiration is to release heat energy.
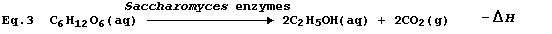
Nevertheless, in most types of respiration, it is true that about 60%
of the energy transduced (DE) is released as heat energy. #
* ATP, which is the immediate source of chemical energy for all living
organisms, provides each living cell with the energy required for its
endergonic processes (e.g., active transport and biosynthesis).
# During their (short-lived) flowering period, Arum lilies carry out a
an alternative respiration process in which all the energy is released
as heat; reproduction is clearly an energy sapping «affair».
Dr. R. Peters Next Contents' List