METALS: COBALT
Cobalt, usually found in the Earth's crust as a sulfide (e.g., in the
ore cobaltite), is the second rarest (0.003%) of the four elements that
can be magnetized; the other three are iron (5.6%), nickel (0.008%),
and gadolinium (0.0005%). This relative rarity means that cobalt is not
used for structural purposes but for the formation of specialized
alloys (e.g., Alnico, which is used to manufacture permanent magnets).
This transition metal, which has a high melting point (1495°C) and a
high density (8.89 g cm-³), forms compounds in several oxidation states
[e.g., (blue) cobalt(II) chloride and (brown) cobalt(III) chloride].
[.. K > Ca > Na > Mg > Al > Zn > Fe > Co > Sn > (H) > Cu > Hg > Ag ..]
1. The extraction of cobalt from its ores is complex, but the final
process usually involves chemical reduction of cobalt(II) dicobalt(III)
oxide (Co3O4). Construct the symbol equation for the reduction of this
tetroxide with each of these reducing agents used in industry:
Aluminium _____________________________________________________________
Carbon ________________________________________________________________
Dihydrogen ____________________________________________________________
[6]
Cobalt can be electrolytically purified, using impure cobalt as the
anode, pure cobalt as the cathode, and aqueous cobalt(II) chloride as
the electrolyte. Explain why solid cobalt(II) chloride would not be
suitable as an electrolyte. ___________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
2. Vitamin-B12, which is synthesized exclusively by bacteria (e.g.,
Methanobacteria), has a structure similar to both chlorophyll-a and
haemoglobin.
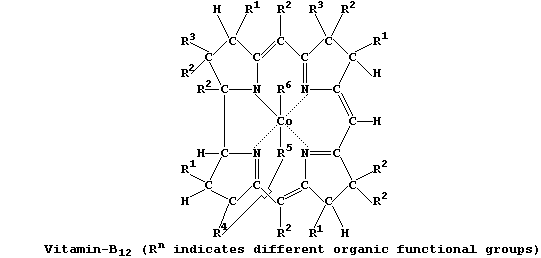
This compound is involved in the bacterial biosynthesis of methane and
organometallic compounds of heavy metals (e.g., dimethylmercury).
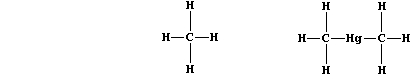
Vitamin-B12 is also a vital cofactor in the synthesis of mammalian red
blood cells; it is acquired from dietary sources and from symbiotic
bacteria residing in the alimentary canal. Name the human deficiency
disease caused by a lack of this vitamin. _____________________________
[1]
3. Cobalt(II) chloride is used as an indicator of water; thus, in the
presence of this compound, blue 'cobalt(II) chloride paper' turns pink:
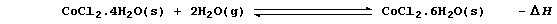
State and explain, using Le Chatelier's Principle, the effect of an
increase in temperature on this reaction. _____________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
4. Anhydrous cobalt(II) chloride is an effective catalyst for the
synthesis of alkanes (in non-aqueous solvents); a typical scheme is: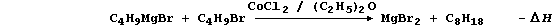
(a) Construct a similar scheme for the synthesis of pentane (C5H12).
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) Complete and label the energy level diagram for this synthesis of
pentane.
Energy ___
___
___________________ _ _ _ _ _ _ _ _ _ _ _ _
___________________
_______________
_______________
___________________________________________
Path of reaction
[5]
(c) Compounds with the same molecular formula but different structural
formulae are known as isomers. Complete these structural formulae to
show the three alkane isomers which have a molecular formula of C5H12.
C C
C C C C C C C C C C C C
C
pentane 2-methylbutane 2,2-dimethylpropane
[3]
(d) Suggest one physical method which could be used to distinguish
between these three isomers. __________________________________________
[1]
5. One radioactive isotope of cobalt, Co-60, has been used in the
treatment of cancer; it has a half-life of 5.3 years; and spontaneously
emits both b-particles and g-rays (i.e., high speed electrons and high
frequency photons, respectively). Determine how many protons, neutrons,
and electrons there are in each of the following.
Co-59 _________________________________________________________________
Co-60 _________________________________________________________________
Co(III)-60 ____________________________________________________________
[3]
Dr. R. Peters Next Contents' List
