METALS: CHROMIUM
Chromium, a relatively rare element in the Earth's crust (0.01%),
occurs mainly in the ore chromite (impure FeCr2O4). This transition
metal, which has a high melting point (1857°C) and a high density
(8.89 g cm-³), forms compounds in several oxidation states [e.g., (red)
chromium(II) ethanoate, (green) chromium(III) sulfate, (brown-black)
chromium(IV) oxide, and (orange) potassium dichromate(VI)].
[.. K > Ca > Na > Mg > Al > Zn > Cr > Fe > Pb > (H) > Cu > Hg > Ag ..]
1. Chromium is produced in two forms: ferrochrome, a carbon-containing
alloy used to manufacture stainless and hard 'chromium' steels; and the
pure metal, which is used to electroplate steel and to manufacture
non-ferrous alloys (e.g., Nichrome).
(a) Ferrochrome is formed when a mixture of chromite, coke, and
limestone is heated in a furnace. Complete the symbol equation below,
so as to summarize the conditions used to obtain this alloy:
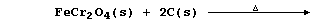
[1]
(b) The extraction of chromium from chromite is complex, but the final
process involves chemical reduction of (green) chromium(III) oxide with
aluminium. Construct the symbol equation for this redox reaction. _____
_______________________________________________________________________
[2]
The metal is electrolytically purified using impure chromium as the
anode, pure chromium as the cathode, and an aqueous chromium salt as
the electrolyte. State the energy change which occurs at the cathode.
_______________________________________________________________________
[2]
(c) Shown below is a diagram of an electrolytic cell used to chromium-
plate a toy-car bumper.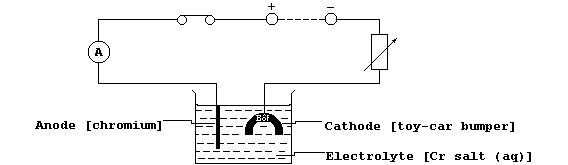
[Q = n × z × F and Q = I × t, where: Q, measured in coulombs (C), is
the quantity of electricity; n is the number of moles of substance
evolved at the electrode; z is the charge on the ion; F is a constant,
with a value of 96500 C mol-¹; I, measured in amps (A), is the current;
and t, measured in seconds (s), is the time.]
The mass (m) of the toy-car bumper increased by 0.0793 g when a direct
current of 1.0 A flowed in the above circuit for 900 s. Calculate the:
Number of moles (n) of chromium deposited at the cathode. _____________
_______________________________________________________________________
Quantity of electricity (Q) which flowed through this circuit. ________
_______________________________________________________________________
Charge (z) on the chromium ion of the electrolyte. ____________________
_______________________________________________________________________
[6]
(d) To prevent rusting, iron objects are sometimes plated with chromium
rather than galvanized with zinc. Suggest one reason why chromium is
used, despite zinc being cheaper and more effective as a sacrificial
anode. ________________________________________________________________
[1]
2. Naturally occurring chromium contains four isotopes: Cr-49 (2.3%),
Cr-50 (4.3%), Cr-52 (83.8%), and Cr-53 (9.6%). Calculate the exact
relative atomic mass of chromium. _____________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
3. Suggest what observations should be made, and construct the symbol
equation, for the reaction of powdered chromium with each of these
reactants [consider chromium(III) to be the preferred oxidation state].
Steam _________________________________________________________________
_______________________________________________________________________
[4]
Aqueous copper(II) sulfate ____________________________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
4. Chromium(III) oxide, an amphoteric substance, is used as a green
pigment in paints and as a catalyst for the polymerization of alkenes.
The pure oxide can be obtained by the thermal decomposition of several
chromium salts; e.g., as can be vividly demonstrated in the 'volcano'
experiment, ammonium dichromate(VI) thermally decomposes as follows: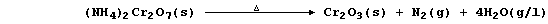
(a) State briefly the meaning of the term 'amphoteric oxide'. _________
_______________________________________________________________________
[1]
(b) Fairly strong heating of anhydrous chromium(III) nitrate results in
the formation of a green solid, a brown gas which turns damp pH paper
red, and a colourless gas which relights a glowing spill. Construct the
symbol equation for the thermal decomposition of chromium(III) nitrate.
_______________________________________________________________________
[2]
5. Dichromates are used extensively as oxidizing agents in industry
and in laboratories; e.g., acidified sodium dichromate(VI) oxidizes
ethanol to ethanoic acid. Complete this scheme to show the structural
formula of the carboxylic acid obtained by oxidizing hexan-1-ol.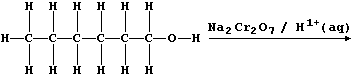
[2]
Hexanoic acid, together with C7, C8, C9, C10, and C12 straight-chain
carboxylic acids, is a component of the trail pheromone of at least one
species of ant (Lasius fuliginosus). Speculate on the purpose of the
'trail'. ______________________________________________________________
[1]
6. Chromium ions are potential pollutants, because the amounts used
industrially are large compared to the ambient concentrations tolerated
by living organisms. These ions are certainly toxic and may also be
carcinogenic: nevertheless, in trace quantities, Cr(III) ions have been
shown to be essential in mammals; e.g., together with the hormones
insulin and glucagon, they help maintain the homeostatic concentration
of glucose in the blood.
(a) In which organ is insulin biosynthesized? _________________________
(b) In which organ does insulin stimulate the conversion of soluble
glucose to insoluble glycogen? ________________________________________
(c) Name one (non-infectious) disease caused by a deficiency of insulin
or chromium(III) ions. ________________________________________________
[3]
Dr. R. Peters Next Contents' List

