METALS: EXTRACTION of COPPER
Copper, which is relatively rare in the Earth's crust (0.006%), occurs
mainly as a sulfide (e.g., the ore chalcopyrite contains CuFeS2). This
metal, which has been shown to be essential to all biological species,
is a typical transition element. Thus, copper has a high melting point
(1083°C) and a high density (8.92 g cm-³), forms coloured compounds
(which are often blue or green), and shows variable oxidation states
[e.g., Cu(I) and Cu(II)]. Furthermore, copper and many of its compounds
show catalytic activity [e.g., copper(II) oxide is used as a catalyst
in the manufacture of methanol, and several copper-containing enzymes
are involved in photosynthesis and in respiration].
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. There are several routine methods for the extraction of copper, but
the processes used, which can be fairly complex, are dependent on both
the composition and quality of the ore. However, the final steps in one
method can be summarized by the following pair of equations:
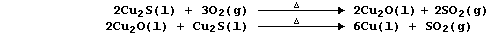
In industry, copper is electrolytically purified using slabs of impure
copper as anodes, thin sheets of pure copper as cathodes, and solutions
of copper(II) sulfate as electrolytes; this industrial process can be
modelled in the laboratory using the electrical circuit shown as Cell 1
in this diagram.
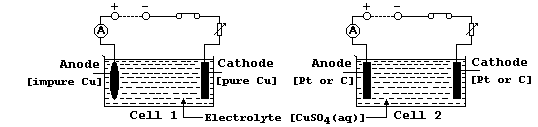
Aqueous copper(II) sulfate contains Cu2+(aq), SO42-(aq), OH1-(aq), and
H1+(aq) ions. When this solution is electrolyzed, the cathodic reaction
always results in copper being deposited, but the anodic reaction is
dependent on the conductor acting as the anode: aqueous copper(II) ions
are formed using a copper anode (Cell 1), whereas oxygen gas is evolved
using either a platinum or carbon-graphite anode (Cell 2).

(c) In cell 1, providing the anode is replaced periodically, continued
electrolysis results in the electrolyte remaining blue-coloured. In
cell 2, by contrast, continued electrolysis results in the electrolyte
becoming colourless [as the concentration of Cu2+(aq) ions decreases].
Suggest what this colourless solution contains. _______________________
[1]
2. Copper is used extensively in water piping, alloys, electroplating,
and in electrical wiring. Suggest two reasons for this widespread use.
_______________________________________________________________________
[2]
3. An industrial chemist investigated the following hypothesis: 'For
the electrolysis of aqueous copper(II) sulfate, the number of moles
(n) of copper deposited at a cathode increases in direct proportion to
time (t); i.e., n = k × t'; the Table shows a summary of the chosen
conditions and raw data.
Constants: aqueous copper(II) sulfate (600 cm³; 0.20 mol dm-³); direct
current (5.35 A); room temperature (17°C); surface area and distance
between copper electrodes (values measured but not recorded).
Time (t) / s |
0 |
60 |
120 |
180 |
180 |
180 |
240 |
300 |
Cu deposited / mg |
0 |
110 |
210 |
250 |
315 |
325 |
430 |
530 |
Cu deposited (n) / mmol |
0.0 |
1.7 |
3.3 |
3.9 |
4.9 |
5.1 |
6.7 |
8.3 |
(a) A value of a dependent variable which does not follow the general
pattern is usually referred to as an 'anomalous result'. In this
investigation, for example, the first value of n when t = 180 s appears
anomalous. Suggest one possible reason for this anomaly. ______________
_______________________________________________________________________
[1]
(b) Noting that the independent variable is on the horizontal axis,
plot all nine data points on this fully labelled graph paper, and then
draw a best straight line through as many points as is sensible.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Cu 8_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
d 7_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
p 6_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
o |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
s 5_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
i |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
t 4_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
d 3_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
(n) 2_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
/ 1_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
mmol 0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | |
0 60 120 180 240 300
Time (t) / s
[3]
This graph shows direct proportionality between the two variables,
because the straight line passes through the origin. Determine the
gradient of the graph; this value, 'k', is the proportionality constant
in the directly proportional relationship n = k × t.
(y2 - y1)
k = ————————— =
(x2 - x1)
[3]
Complete this precisely worded conclusion (which incorporates the
constants). 'When a direct current of _______ was passed through an
electrolysis cell containing _______ electrodes and 600 cm³ of aqueous
copper(II) sulfate (0.20 mol dm-³), at room temperature (17°C), the
number of moles (n) of copper deposited at the cathode increased in
direct proportion to the time (t) of the electrolysis (within the
range __________); i.e., n = k × t, where k = ________________.'
[4]
Dr. R. Peters Next Contents' List

