METALS: COPPER COMPOUNDS (1)
Most reactions of transition metal compounds can be usefully classified
into two types. First, those known as ligand-exchange reactions, which
involve the replacement of one or more ligands (coordinatively) bonded
to a central metal atom or ion; e.g.,

And second, those known as redox reactions, which involve changes in
oxidation states; e.g.,
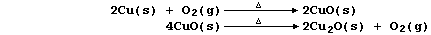
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. In a crystalline lattice there are strong electrostatic forces of
attraction between oppositely charged ions; these bonds are broken when
an ionic solute dissolves in the solvent water, and new ones are formed
in the aqueous solution (i.e., those between water molecules and ions).
Nonetheless, dissolution is usually presented as a simple physical
change ...
(a) Anhydrous copper(II) sulfate, CuSO4(s), is an off-white solid; it
dissolves in water to form aqueous copper(II) sulfate, CuSO4(aq),
which is a blue solution:
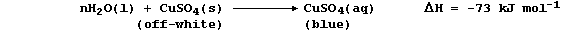
Suggest and explain one reason why this dissolution should be regarded
as a chemical change. _________________________________________________
_______________________________________________________________________
[2]
(b) Aqueous copper(II) sulfate is a mixture of Cu2+(aq), SO42-(aq),
H1+(aq), and OH1+(aq) ions. State the formulae of the two ions that are
present in (anhydrous) copper(II) sulfate, but not in this mixture.
_______________________________________________________________________
[2]
(c) Strong heating of aqueous copper(II) sulfate, in a distillation
apparatus, results in the quantitative recovery of copper(II) sulfate,
as an off-white solid, and water, as a colourless liquid: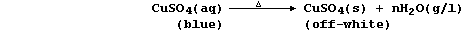
By contrast, slow evaporation of aqueous copper(II) sulfate results in
the formation of hydrated copper(II) sulfate as blue crystals:

State why both of these evaporative processes, nominally designated as
'physical', should be regarded as chemical changes. ___________________
_______________________________________________________________________
[1]
Name one dehydrating agent which can effect the dehydration of hydrated
copper(II) sulfate. ___________________________________________________
[1]
2. Copper, a poorer reducing agent than hydrogen, is not oxidized by
H1+(aq): so, in contrast to the metals above hydrogen in the reactivity
series, copper does not react with water, steam, or dilute acids; i.e.,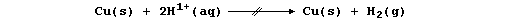
Nevertheless, copper can be oxidized by both concentrated sulfuric and
nitric acids; the oxidizing agents are, respectively, SO42-(aq) and
NO31-(aq). Construct a symbol equation for the oxidation of copper by:
Concentrated sulfuric acid (Mr of the gaseous product is 64). ________
_______________________________________________________________________
Concentrated nitric acid (Mr of the gaseous product is 46). ___________
_______________________________________________________________________
[4]
3. Copper(II) oxide can be reduced to copper by a number of reducing
agents, including more reactive elements; e.g.,
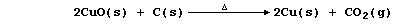
Construct a symbol equation for the reduction of copper(II) oxide with:
Ammonia (molar masses of the gaseous products are 28 and 18 g mol-น).
_______________________________________________________________________
Propane (molar masses of the gaseous products are 44 and 18 g mol-น).
_______________________________________________________________________
[4]
4. Probably the most familiar example of a copper(I) compound is that
observed when a positive result is obtained in the Fehling's test for
'reducing sugars'; e.g., warming an alkaline solution of glucose and
copper(II) sulfate results in the precipitation of copper(I) oxide as
a pale-orange solid. Suggest why a carbohydrate which gives a positive
result in this test is known as a reducing sugar. _____________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Name one other reducing sugar. ________________________________________
[1]
5. Aqueous solutions of copper(I) ions are unstable with respect to
aqueous copper(II) ions and copper; i.e.,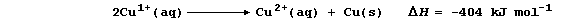
A blue solution and a brown precipitate are formed when copper(I) oxide
is warmed with dilute sulfuric acid. Construct the symbol equation for
this redox reaction. __________________________________________________
_______________________________________________________________________
[2]
6. Ethene is the simplest plant growth regulator known; it stimulates
the lateral expansion of elongating cells and promotes fruit ripening
and leaf drop. Researchers have synthesized several copper(I) compounds
which appear to be reasonably suitable models for the 'binding site' of
ethene; shown below is the structural formula of a typical example. [In
animals, the nearest equivalent of a binding site is the receptor site
associated with a chemical synapse (where neurotransmitters such as
acetylcholine and dopamine are released)].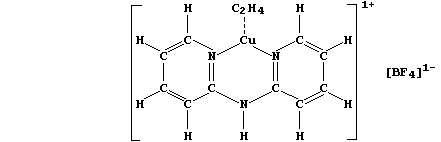
Using patterns inherent in the Periodic Table and/or organic chemistry,
draw the structural formulae of two copper(I) analogues of the above
model compound; for clarity, omit the anions. _________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Dr. R. Peters Next Contents' List