METALS: COPPER COMPOUNDS (2)
Metal ions are regarded as pollutants when, as a direct or indirect
result of Man's activities, their concentrations in any given ecosystem
increase. Popular attention invariably focuses on the toxic effects of
ions which have little or no biological rôle; e.g., those of aluminium,
cadmium, lead, and mercury. However, a correctly balanced perspective
of pollution also requires an awareness of the potential toxicity of
biologically essential ions; e.g., those of copper, iron, manganese,
and zinc. Thus, although each species has evolved mechanisms of using,
and adapting to, molecules and ions at their ambient concentrations,
adverse effects occur at higher concentrations because such homeostatic
mechanisms are overwhelmed. Increased concentrations of hydrated metal
ions in the environment are most commonly attributable to: the use of
biocides; leaching from active or derelict mines; the illegal discharge
of industrial waste; and, the products of neutralization reactions
between the components of 'acid rain' and metal ores.
1. Various copper(II) compounds, including the ethanoate, hydroxide,
and sulphate, have been used as fungicides and as insecticides; this
scheme summarizes their syntheses via neutralization and precipitation
reactions.
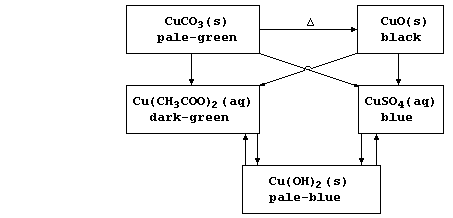
(a) Construct the symbol equation for the neutralization of:
Dilute ethanoic acid with copper(II) carbonate ________________________
_______________________________________________________________________
Dilute sulfuric acid with copper(II) oxide ____________________________
_______________________________________________________________________
[2]
Complete the following description of one method of preparing crystals
of copper(II) sulfate pentahydrate. "Wear safety glasses. To 25 cm³ of
gently-boiling dilute sulfuric acid, add copper(II) oxide in small
portions until the reaction mixture no longer turns universal indicator
paper ____. Filter this mixture, _________ evaporate the filtrate using
a boiling water-bath, and then leave the blue solution to _____________
at room temperature in a labelled petri dish covered with paper."
[3]
(b) Construct the net ionic equation for the precipitation reaction
summarized by this symbol equation: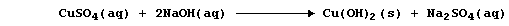
_______________________________________________________________________
[1]
Complete the following description of one method of preparing a dry
sample of copper(II) hydroxide. "Wear safety glasses and _______. Use a
burette to add 5.0 cm³ of aqueous copper(II) sulfate (1.00 mol dm-³)
into a test-tube. Use a separate burette to add carefully _________ of
aqueous sodium hydroxide (1.00 mol dm-³) to the blue solution in the
test-tube. Shake the mixture thoroughly, centrifuge, and then decant
the colourless supernatant. Add about 15 cm³ of __________ water to the
precipitate, and then repeat the shaking, centrifuging, and decanting
steps. Leave the precipitate to dry in a labelled petri dish."
[3]
2. A number of grass species, including Agrostis tenuis, have evolved
tolerance to certain metal ions; e.g., those of copper, lead, and zinc.
A partial explanation of the evolution of a grass species tolerant to
high concentrations of copper(II) ions is included below.
(a) A mutation is the spontaneous change in the structure of DNA or of
RNA, involving either a whole chromosome or an allele (i.e., a sequence
of nucleotides coding for one polypeptide). Mutations occur naturally
all the time, but various types of radiation (and chemicals) increase
their frequency. Name one type of radiation which increases mutation
rates. ________________________________________________________________
[1]
(b) A species adapts to ambient concentrations of copper(II) ions, and
so homozygous recessive (tt) parents will produce offspring which
show no tolerance to excess copper(II) ions. However, when a favourable
mutation occurs in the reproductive cells of one grass plant, resulting
in the formation of a dominant allele (T) that codes for tolerance,
then its genotype becomes Tt and its phenotype 'tolerant'. As this
first genetic diagram shows, 50% of the offspring produced by this
heterozygous (Tt) parent and a homozygous recessive (tt) parent will be
'tolerant'; these will be the fittest in an environment where the agent
of selection is a high concentration of copper(II) ions, and so be the
most likely to survive to reproductive maturity.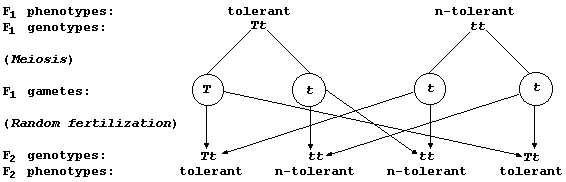
Construct a second genetic diagram to show the genotypes and phenotypes
of the offspring produced by parents who are both heterozygous. _______
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
State the genotype and phenotype of all the offspring produced by a
homozygous dominant (TT) parent who reproduces by self-fertilization.
_______________________________________________________________________
[2]
(c) Tolerant grass species are now used in industrial regions to turn
derelict mines and slag heaps into recreational areas. Recalling that
these autotrophs are the producers in diverse food chains, suggest and
explain one possible ecological disadvantage of this attractive method
of conservation. ______________________________________________________
_______________________________________________________________________
[2]
Dr. R. Peters Next Contents' List
