ORGANIC CHEMISTRY: DETERGENTS
Washing products used to simplify the chore of cleaning clothes include
soaps and detergents. Most soaps are sodium salts of the long-chain
carboxylic acids obtained by hydrolysis of animal fats and plant oils;
and the structural formula of a typical soap, sodium octadecanoate, is:
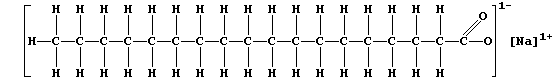
Currently, for most domestic and industrial applications, the preferred
products are the synthetic detergents manufactured from petroleum oil
fractions. This reaction scheme summarizes the synthesis of a typical
detergent, sodium 4-dodecylbenzenesulfonate.
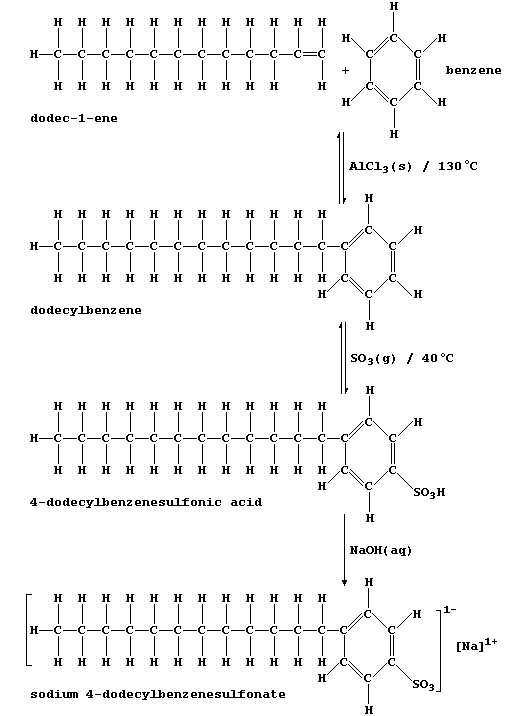
1. Dodec-1-ene and benzene are usually obtained via catalytic cracking
of petroleum oil fractions; e.g., cracking of hexadecane, C16H34, can
produce this straight-chain isomer of dodecene and one other molecule.
(a) Construct the symbol equation for this cracking process. __________
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) Suggest what would be observed if a mixture of alkaline potassium
manganate(VII) and dodec-1-ene was shaken. ____________________________
_______________________________________________________________________
[2]
(c) Details of cracking processes, which often involve co-catalysts
such as Pt-Al2O3, are closely guarded industrial secrets. Nevertheless,
published research indicates that such processes must require careful
control because catalytic isomerization results in branched isomers
being formed. Draw one branched-chain isomer of dodecene. _____________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(d) Detergents made using branched-chain alkenes are not biodegradable.
Explain the meaning of the term 'biodegradable'. ______________________
_______________________________________________________________________
[2]
Suggest one reason why non-biodegradable detergents are considered to
be pollutants. ________________________________________________________
[1]
2. The addition of benzene across the double bond of dodec-1-ene, to
form dodecylbenzene, is analogous to the addition of bromine to ethene.
Predict the name, draw the structural formula, and calculate the molar
mass of the compound formed by the addition of benzene to ethene. _____
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
3. Suggest one advantage in using a low reaction temperature for the
substitution reaction of sulfur trioxide with dodecylbenzene. _________
_______________________________________________________________________
[1]
4. The neutralization of 4-dodecylbenzenesulfonic acid is normally
carried out with sodium hydroxide. Name a less corrosive alkali which
should be suitable for this reaction. _________________________________
[1]
5. The efficacy of detergents is only slightly reduced if hard water
is used, because Group 2 alkylbenzenesulfonates are soluble; i.e., in
contrast to the insoluble Group 2 salts of long-chain carboxylic acids,
precipitates of 'scum' are not produced. Give the formulae of two
substances which cause hardness. ______________________________________
[2]
Dr. R. Peters Next Contents' List
