METALS: DISPLACEMENT REACTIONS (1)
From a consideration of a reactivity series based on redox potentials,
one might reasonably predict that a metal should displace hydrogen from
either water or dilute acids if it is more reactive than hydrogen ...
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
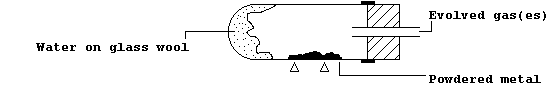
1. The Table summarizes the results of experiments which examined the
reactions of eleven metals with water and/or steam, whereas the diagram
shows the apparatus used for the experiments with steam.
| Observations of reactions of metals with water and/or steam * |
K |
Violent reaction with cold water; metal floated on water as
a small molten ball; evolved gas burnt spontaneously with a
lilac flame; resulting solution turned universal indicator
paper blue-purple. |
Ca |
Very vigorous reaction with cold water; evolved gas produced
an explosive pop with a lighted splint; resulting milky-white
suspension, which was very hot, turned universal indicator
paper dark-blue. |
Na |
Very vigorous reaction with cold water; metal floated on water
as a small molten ball; evolved gas did not burn spontaneously,
but produced an explosive pop with a lighted splint; resulting
solution turned universal indicator paper blue-purple. |
Mg |
Little or no reaction with cold water. Very slow reaction with
hot water (containing traces of universal indicator solution);
the solution changed slowly from green to blue over a period
of two hours (but no precipitate formed). Rapid reaction with
steam; white solid formed; evolved gas burnt. |
Al |
No reaction with boiling water: although reaction with boiling
water containing a catalytic quantity of sodium chloride;
evolved gas burnt with an explosive pop. Fairly rapid reaction
with steam; white solid formed; evolved gas burnt. |
Zn |
No reaction with boiling water. Fairly slow reaction with
steam; initially formed yellow solid cooled to a white solid;
evolved gas burnt intermittently. |
Fe |
Very slow reaction with steam; black solid formed; gas, if
evolved, not in sufficient quantity to be burnt. |
Sn |
Little or no reaction with steam; trace of white solid formed. |
Pb |
Little or no reaction with steam; trace of white solid formed. |
Cu |
No (evidence for) reaction with steam. |
Ag |
No (evidence for) reaction with steam. |
* Caveat This Table presents descriptors which indicate both relative
reaction speeds & energy changes; this conflation must not be allowed
to obscure the fact that there is no connection between the activation
energy & the heat energy change (DH) for a chemical reaction.
|
(a) In common with similar presentations in textbooks, these results
do not refer to controlled experiments. Thus, although the qualitative
independent variable chosen was 'metal', no attempts were made to keep
other variables constant; e.g., the volume of water or the absence of
catalysts. Name the two physical quantities of the metals which should
have been held constant. ______________________________________________
[2]
(b) The symbol equation for the reaction between potassium and water is
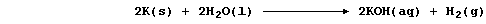
Noting that calcium and sodium react with water similarly, construct
the symbol equation for the reaction between calcium and water. _______
_______________________________________________________________________
[2]
Calcium hydroxide is an alkali, because it is a base which is soluble
in water (albeit only slightly soluble). What is the common name for
aqueous calcium hydroxide? ____________________________________________
[1]
(c) Magnesium reacts with steam to form the hydroxide,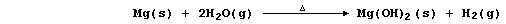
but this thermally decomposes at the temperature of the experiment,
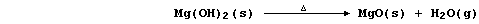
so the reaction between magnesium and steam is usually summarized as:
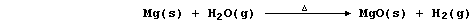
Noting that aluminium and zinc react with steam similarly, construct
the (overall) symbol equation for the reaction between zinc and steam.
_______________________________________________________________________
[1]
(d) Iron has been shown to react reversibly with steam; i.e.,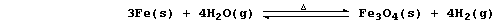
Note, however, that these particular experiments could not determine
the reversible character of these reactions, because the apparatus used
was an 'open system'. Name one substance which is transferred to the
surroundings in these experiments. ____________________________________
[1]
[Neither tin nor lead react with water or steam: but both react with
dilute acids. The explanation for these observations is, regrettably,
beyond the scope of this text; nevertheless, one should be aware that
redox potentials are dependent on pH.]
2. Readily duplicated investigations have shown that, under comparable
conditions, metals react faster with dilute acids than with water; a
partial explanation for this difference is as follows. Water undergoes
very slight dissociation (or ionization); i.e.,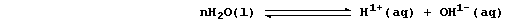
Because the position of the above equilibrium lies almost completely to
the left, the concentration of hydrogen ions in water is low [roughly
10-7 mol dm-³ (i.e., pH 7)]. By contrast, dilute acids undergo either
partial or complete dissociation; e.g.,
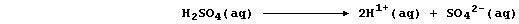
Therefore, the concentration of hydrogen ions in dilute acids is higher
[ranging from 10-1 to 10-6 mol dm-³ (pH 1 to 6)]. In consequence, there
will be more collisions between metal atoms and these ions in dilute
acids than in water, and so faster reactions.
Construct the symbol equation for the reaction between:
Sodium and dilute hydrochloric acid (dangerously explosive) ___________
_______________________________________________________________________
Magnesium and dilute sulfuric acid (vigorous) _________________________
_______________________________________________________________________
Aluminium and dilute sulfuric acid (fairly vigorous) __________________
_______________________________________________________________________
Iron and dilute sulfuric acid (which, in part, is analogous to that
between steel and this component of 'acid rain') ______________________
_______________________________________________________________________
[8]
3. Neither copper nor silver react with water, steam, or dilute acids.
Name another metal which is similarly unreactive. _____________________
[1]
Dr. R. Peters Next Contents' List