METALS: DISPLACEMENT REACTIONS (2)
A displacement reaction is the chemical change which occurs when a more
reactive element displaces a less reactive element from its compound;
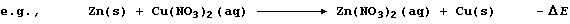
In this redox reaction, electrons are transferred from zinc atoms to
copper(II) ions because zinc is a better reducing agent (i.e., it is
more easily oxidized); the redox half equations are:
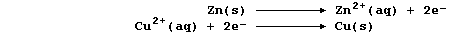
The aqueous nitrate ions are spectators, so the net ionic equation is:
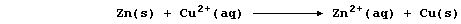
[.. K > Sr > Ca > Mg > Be > Al > Zn > Fe > Sn > (H) > Cu > Hg > Ag ..]
1. Temperature rises (DT) were measured for displacement reactions
involving beryllium and aqueous solutions of metal nitrates; this first
Table shows a summary of the chosen conditions and raw data.
Constants: volume (25 cm³), concentration (0.25 mol dm-³), and starting
temperature (17°C) of aqueous nitrate; beryllium powder (an excess);
thermometer (0-100°C); reaction vessel (an insulated plastic cup).
Nitrate(aq) |
Mg(II) |
Mg(II) |
Al(III) |
Zn(II) |
Pb(II) |
Cu(II) |
DT / °C |
0 |
0 |
6 |
14 |
32 |
45 |
(a) Beryllium compounds are extremely toxic. Apart from wearing safety
glasses, suggest one other vital safety precaution adopted for these
experiments. __________________________________________________________
[1]
(b) For the reaction between beryllium and aqueous copper(II) nitrate,
construct the symbol equation and state two other observable changes.
_______________________________________________________________________
_______________________________________________________________________
[3]
(c) State why no temperature rise was observed with aqueous magnesium
nitrate. ______________________________________________________________
[1]
(d) Use the results in this first Table to predict the temperature rise
(DT) that would be obtained, using the same set of constants, with
these aqueous nitrates: Sr(II) _______ Fe(II) _______ Ag(I) _______
[3]
2. A research chemist investigated the following hypothesis: 'The
speed (S) of reaction between beryllium and aqueous hydrochloric acid
increases in direct proportion to the concentration (C) of the acid;
i.e., S = k × C'; this second Table shows a summary of the chosen
conditions and raw data.
Constants: length (6.0 cm) and surface area (6.0 cm²) of beryllium
ribbon; volume of solution (25 cm³); room temperature (21°C); absence
of catalysts; volume of dihydrogen collected via gas syringe (25 cm³).
Concentration (C) / mol dm-³ |
0.0 |
0.0 |
0.15 |
0.30 |
0.45 |
0.60 |
0.75 |
Reaction time (t) / s |
»900 |
»900 |
120 |
58 |
42 |
32 |
25 |
Reaction speed (S) / ms-¹ |
0.0 |
0.0 |
|
|
|
|
40 |
(a) Construct the net ionic equation for the displacement reaction
investigated in the above hypothesis. _________________________________
_______________________________________________________________________
[2]
(b) Calculate the missing values for the reaction speed, and insert
these data in this second Table above.
[2]
(c) Label both axes, and then plot all seven data points.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
40_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
30_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
20_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_| Beryllium |_|_|_|_|_|_|_|_|_|_|_|
10_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | | |
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7
[4]
Draw a best straight line through as many points as is sensible, and
then determine the gradient of the (beryllium) graph; this value, 'k',
is the proportionality constant in the directly proportional
relationship S = k × C. _______________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Write a precisely worded conclusion based on the (beryllium) graph.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[5]
(d) Strictly speaking, to extrapolate a relationship outside the range
of the independent variable examined is scientifically flawed: but to
do so has practical advantages with respect to safety considerations,
time, and costs. Use the equation, and your value of 'k', to estimate
the reaction time if concentrated hydrochloric acid was used (i.e.,
C = 10 mol dm-³; pH -1). ______________________________________________
_______________________________________________________________________
[2]
(e) Sketch the graphs that would be obtained if Be was replaced with Ca
and with Ag. Recalling that there is no connection between the energy
change and the activation energy, suggest and explain using a symbol
equation, why each graph differs from the one obtained for Be.
Calcium _______________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
Silver ________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
Dr. R. Peters Next Contents' List