METALS: DISPLACEMENT REACTIONS (4)
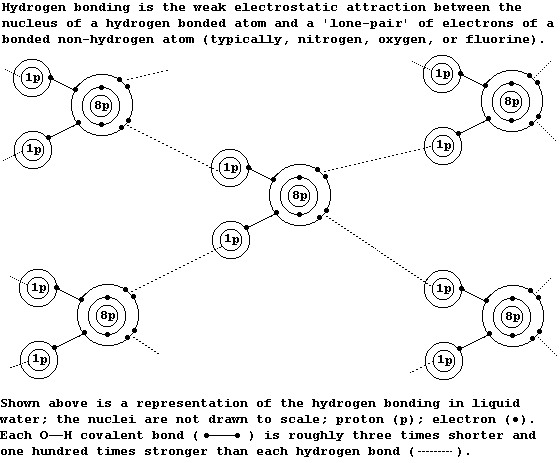
Pure water is a covalently bonded compound with some unusual physical
properties; these include a high boiling point, a high surface tension,
a maximum density as a liquid, a high latent heat of vaporization, and
the highest heat capacity of any liquid. All of these properties are
partially attributable to water's extended structure, which involves
'hydrogen bonding'; this type of bonding is present in many molecules,
(for example, and in particular, between the strands of DNA double
helices), but its extent in pure water appears to be truly exceptional.
However, pure water is rarely encountered in Nature because it is such
an excellent solvent in which many solutes dissolve to varying degrees.
Furthermore, the resulting aqueous solutions might quite reasonably be
expected to have different properties; e.g., heat capacities ...
1. A research chemist decided to use a metal displacement reaction,
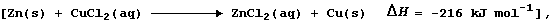
to examine this hypothesis: 'As the concentration (C) of chloride ions
increases, the specific heat capacities (c) of the aqueous solutions
decrease in linear proportion; i.e., c = k × C + i'; the Table shows a
summary of the chosen reaction conditions and raw data.
Constants: heat energy supplied (H = 0.540 kJ); powdered zinc (excess);
aqueous copper(II) chloride (0.25 mol dm-³; 10.0 cm³ via bulb pipette);
starting temperature of reaction mixture (20.0°C ± 0.2°C); thermometer
(0-50°C, graduated to 0.1°C); reaction vessel (thermos flask).
NaCl(s)
added /
g |
Concentration
of Cl1- (C) /
mol dm-³ |
Density
r /
kg dm-³ |
Total mass
m /
kg |
Temperature
rise (DT) /
K |
Specific heat
capacity (c) /
kJ kg-¹ K-¹ |
0.00 |
0.50 |
1.02 |
0.0102 |
13.8 |
|
0.59 |
1.50 |
1.05 |
0.0105 |
14.9 |
|
1.17 |
2.50 |
1.08 |
0.0108 |
14.8 |
3.38 |
1.17 |
2.50 |
1.08 |
0.0108 |
15.3 |
3.27 |
1.17 |
2.50 |
1.08 |
0.0108 |
15.5 |
3.25 |
1.76 |
3.50 |
1.11 |
0.0110 |
15.7 |
|
2.34 |
4.50 |
1.14 |
0.0113 |
15.7 |
|
(a) The standard equation H = m × c × DT rearranges to c = H ÷ m × DT.
Using this rearranged equation, calculate the missing values for the
specific heat capacity (c), and insert these data in the Table.
[3]
(b) The first specific heat capacity for C = 2.50 mol dm-³ appears to
be an anomalous result. Suggest one possible reason for this anomaly.
_______________________________________________________________________
[1]
(c) Label both axes, plot all seven data points, and then draw a best
curve through as many points as is sensible.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
3.8_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3.6_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3.4_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3.2_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3.0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0.0_/_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | | | | |
0.0 1.0 2.0 3.0 4.0
[5]
Complete this precisely worded conclusion, based on the tabulated and
graphical data. 'As determined from displacement reactions at room
temperature (20°C), using excess zinc powder and 10.0 cm³ of aqueous
copper(II) chloride (0.25 mol dm-³), specific heat capacities (c) of
aqueous solutions of sodium chloride decreased as the concentration (C)
of chloride ions __________ (within the range ___________________). The
curve indicates that these two variables are not in ___________________
to each other; i.e., ________________'
[4]
(d) Here, unfortunately, values of the dependent variable outside the
range of the independent variable examined cannot be obtained either
by calculation, because of the non-linear relationship between the
variables, or by extrapolation, because of the graph's limited range.
Nevertheless, from inspection of the tabulated data, guesstimate a
value for the specific heat capacity (c) of saturated aqueous sodium
chloride (C = 6.5 mol dm-³). __________________________________________
[1]
(e) Complete the following paragraph, which incorporates one partial
interpretation and one possible consequence of the results of the above
investigation. 'The specific heat capacity of an aqueous solution
decreases as the concentration of chloride ions __________, presumably
because the amount of hydrogen bonding __________. In consequence, its
ability to act as a heat buffer, in intra-cellular or extra-cellular
environments, will _________; furthermore, because all biochemical
reactions are controlled by temperature-sensitive enzymes, this may
result in __________ efficiency of these biological catalysts.'
[4]
Dr. R. Peters Next Contents' List