EXPERIMENTAL: PREPARATION OF WATER-SOLUBLE SALTS USING NEUTRALIZATION
AND METAL DISPLACEMENT REACTIONS
Introduction
Reactions used to prepare salts, which are ionic compounds made up of
ions other than H1+, OH1-, or O2-, include neutralization (i.e., the
reaction between an acid and a base), displacement (i.e., when a more
reactive element displaces a less reactive element from its compound),
and precipitation (i.e., when two soluble ionic compounds react to form
a precipitate). Shown below are equations which summarize exemplars of
these three reaction types; neutralization (1 - 4), displacement (5 and
6), and precipitation (7).
(1) NiO(s) + 2HCl(aq) ——————————————® NiCl2(aq) + H2O(l)
(2) Mg(OH)2(s) + H2SO4(aq) ——————————® MgSO4(aq) + 2H2O(l)
(3) PbCO3(s) + 2HNO3(aq) ————————————® Pb(NO3)2(aq) + H2O(l) + CO2(g)
(4) 2NH3(aq) + H33PO4(aq) ———————————® (NH4)2HPO4(aq)
(5) Zn(s) + H2SO4(aq) ———————————————® ZnSO4(aq) + H2(g)
(6) 2Al(s) + 3Zn(NO3)2(aq) ——————————® 2Al(NO3)3(aq) + 3Zn(s)
(7) 2KOH(aq) + FeSO4(s) —————————————® 2Fe(OH)2(s) + K2SO4(aq)
Whereas a precipitation reaction is commonly used for the preparation
of an insoluble salt, neutralization and metal displacement reactions
are often those of choice for the preparation of water-soluble salts.
[... K > Ca > Na > Mg > Al > Mn > Zn > Fe > Co > Pb > (H) > Cu > Ag ..]
First, using a neutralization reaction, you are required to prepare
crystals of either copper(II) ethanoate or copper(II) chloride, using
appropriate starting materials from: aqueous ethanoic acid; aqueous
sulfuric acid; aqueous hydrochloric acid; copper; copper(II) oxide;
copper(II) carbonate; copper(II) sulfate; and copper(II) nitrate.
And second, using a metal displacement reaction, you are required to
prepare crystals of a second ethanoate or chloride. One starting
material should be some of your freshly prepared copper(II) ethanoate
or copper(II) chloride; and the other should be a suitably correct
choice from: cobalt; cobalt(II) carbonate; manganese; manganese(III)
carbonate; silver; silver(I) carbonate; zinc; and zinc carbonate. |
Before starting your experimental work, be sure to complete this flow
chart - which should summarize your choice of reactants and products.
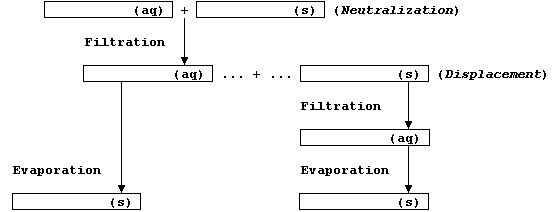
Method (1) - via a Neutralization Reaction
1. Collect the following (for Methods 1 and 2): safety glasses; Bunsen
burner kit; 100 cm³ glass beaker; measuring cylinder (25 cm³); stirring
rod; filter funnel; 2 filter papers; retort stand and clamp; 2 splints;
evaporating dish; 2 petri dishes; pH paper; and starting materials.
2. Set up, ready for use, the Bunsen burner kit and the filtration
apparatus; until required, the evaporation and petri dishes should be
lying face downwards on tissue paper.
3. Using the measuring cylinder, place 25 cm³ of dilute acid into the
beaker equipped with a stirring rod; check the pH of the acid.
4. Warm the acid using a small Bunsen flame (or a boiling water-bath
if a metal is used as one of the starting materials).
5. [Throughout this step, you should be prepared to switch off the
Bunsen if the reaction becomes too vigorous. Furthermore, you should
try and maintain a volume of about 25 cm³ for the reaction mixture, by
adding small volumes of water as and when required.] To the warm acid,
add the solid starting material in small portions until it is in slight
excess; that is, until no more dissolves and the pH paper no longer
turns red.
6. [The reaction mixture contains an excess of insoluble starting
material and the required salt dissolved in water.] Dampen the filter
paper and, using the stirring rod as a guide, filter the warm reaction
mixture into the evaporating dish; it is good technique to wash both
the beaker and filter paper with a little water.
7. [The filtrate (and washings) should contain only the required salt
dissolved in water.] Transfer half the filtrate to a petri dish; cover
this dish with paper, (labelled with name, contents, and date), and
allow the solution to crystallize at room temperature.
8. Using Sellotape, attach a small sample of these crystals to this
worksheet; to be safe, you must assume the compound is toxic.
Method (2) - via a Displacement Reaction
1. To the second half of the filtrate (see step 7 above), stir in the
metal powder in small portions until in slight excess; that is, until
there are no more changes to the supernatant.
2. Dampen the filter paper and, using the stirring rod as a guide,
filter this reaction mixture into a second petri dish ....
3. Cover this dish with paper, (labelled with name, contents, and
date), and then allow the solution to crystallize at room temperature.
4. Using Sellotape ............ you must assume the compound is toxic.
Notes
Method 1 can be used to prepare most water-soluble salts, providing the
solid starting material, (i.e., metal, oxide, hydroxide, or carbonate),
is insoluble in water. However, a 'titration' method must be used if
the starting material is soluble in water (i.e., all Group 1 compounds,
ammonium compounds, nitrates, and ethanoates).
Dr. R. Peters Next Contents' List
