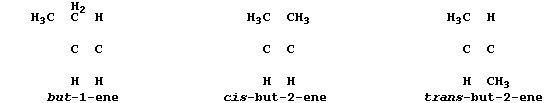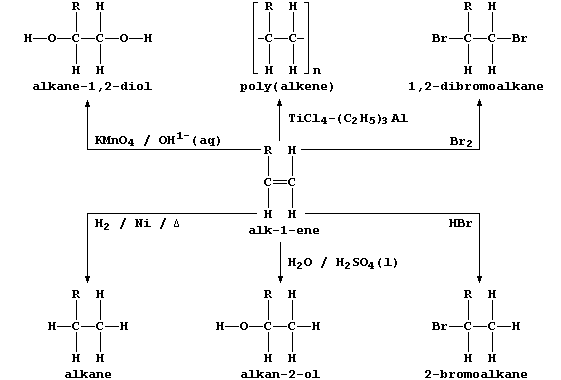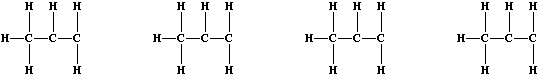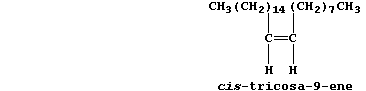ORGANIC CHEMISTRY: ALKENES
All chain alkenes belong to an homologous series which has the general
formula CnH2n; and all - except ethene and propene - can occur in two
or more isomeric forms (as evinced by molecular models). In industry,
many of these unsaturated hydrocarbons are obtained by the fractional
distillation of products formed by cracking petroleum oil fractions.
Alkenes are used as synthetic intermediates; this use, as well as their
versatility, is attributable to the wide variety of reagents which add
across the reactive double bond present in every alkene.
1. Complete these structural formulae to show the three alkene isomers
which have the molecular formula of C4H8.
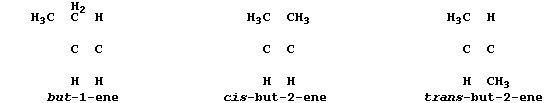
[2]
2. This general scheme summarizes some typical addition reactions of
alk-1-enes, where R may be CH3, C2H5, C3H7, C4H9, ...
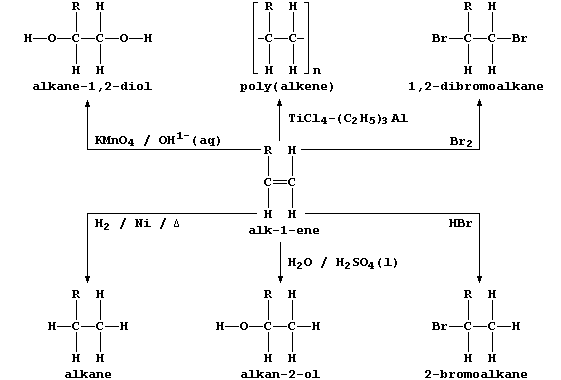
In principle, and often so in practice, the addition of a reagent to an
alkene can result in the formation of isomers; e.g., the addition of
water and of hydrogen bromide to but-1-ene, R = C2H5, could also give
butan-1-ol and 1-bromobutane, respectively. Draw the full structural
formulae of 1-bromobutane and 2-bromobutane. __________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
3. Isomerism can be considered to represent, Janus-like, 'the beauty
and the bane' of Chemistry ... Thus, on the one hand, isomers often
show important differences in chemical and biological properties; e.g.,
acidified potassium dichromate(VI) oxidizes butan-1-ol, a highly toxic
industrial solvent, but has no effect on ethoxyethane, a sparingly used
anaesthetic popularly known as 'ether'.

However, on the other hand, mixtures of isomers require separation by
physical methods that are often costly in terms of time and/or energy.
Name three physical methods used to separate mixtures. ________________
_______________________________________________________________________
[3]
4. A number of solvents are manufactured by the addition reactions of
halogens with alkenes; e.g., dichlorine reacts with all three butenes
to form dichlorobutanes. Recalling that naturally occurring chlorine
consists of two isotopes, (Cl-35 and Cl-37), complete these structural
formulae to show the four isotopic variants of 1,2-dichloropropane.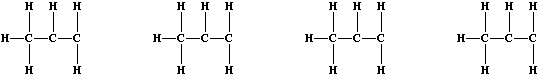
[2]
5. Polymerization is a process whereby thousands of monomer molecules
react to form one polymer molecule; and, as indicated by the general
equation below, alkene monomers undergo addition polymerization:

Calculate the molar mass of the monomeric unit in Perspex, correctly
named as poly(2-methylpropenoate); i.e., R° = CH3, R¹ = H, R² = CO2CH3
and R³ = H. ___________________________________________________________
[2]
6. The major sex pheromone of the female housefly (Musca domestica) is
cis-tricosa-9-ene; its primary function seems to be sexual stimulation
of the males.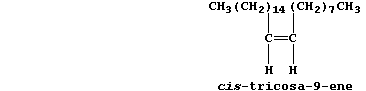
Determine the molecular and empirical formulae of cis-tricosa-9-ene.
_______________________________________________________________________
Name one isomer which would be less likely to behave as a pheromone in
this species. _________________________________________________________
[3]
Dr. R. Peters Next Contents' List