SELECTED PRINCIPLES: EQUILIBRIA (2) In economic terms, it is essential to achieve - in a safe environment - the maximum yield in any given industrial process in the minimum time. Not surprisingly, therefore, the variables which affect the yield and the rate of a reversible reaction are important considerations in plant design; and, for example, certainly would have been in the optimization of one industrial method of manufacturing ethanoic acid, which involves the catalyzed, gas-phase reaction between methanol and carbon monoxide.
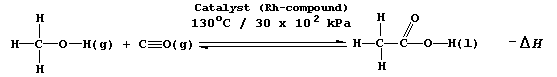
[Scene. A tea-party in the plush boardroom of Hydragyrum Chapelier
Vinaigrette (a small company which, most curiously, manufactures hats
and ethanoic acid); the chairman's name is Monsieur Oliver Scrooge.]
O. Scrooge: We cannot rest on our laurels of the past. So, I want
more, and I want it faster! [The board members ignore
this party pooper.] Otherwise, ... each of you will be
collecting your Christmas present from the Government.
[With this veiled threat, Les Femmes are all attention.]
La Vitesse: Well, ... a catalyst would increase the rate. However,
it is a compound of a precious metal: ... so there would
be a major capital cost. Nevertheless, we can re-use
the catalyst ... providing, of course, we minimize the
introduction of inhibitors into the reaction vessels.
O. Scrooge: Will it increase the yield? [His tone is hopeful.]
La Vitesse: Certainly not! [S. looks glum.] No catalyst changes
the position of equilibrium. On the other hand, without
a catalyst, we would definitely need to use much higher
temperatures: ... so, indirectly, it would reduce costs.
La Chaleur: True, though a high temperature would increase the rate.
O. Scrooge: Yes, ... but will a high temperature increase the yield?
La Chaleur: Certainly not! Do pay attention! The reaction is
exothermic. [S. looks chastened and even more glum.]
La Pression: A high pressure would also increase the rate ... though,
again, with increased operating costs.
O. Scrooge: Yes, yes, ... [His tone is weary.] ... but will a high
pressure increase the yield?
La Pression: Certainly! [S. perks up.] To echo La Chaleur, do pay
attention! Note that there are fewer moles of gas on
the product side of the equation ... well, none in fact.
O. Scrooge: How absolutely splendid! I suggest that we should be
miserly with the catalyst, use a lowish temperature, and
boost the pressure massively. [His tone is bullish.]
La Securité: No, ... not necessarily. I must urge caution. Reaction
vessels strong enough to withstand very high pressures
are exceedingly expensive. The safety of our workers
and the general public is paramount!
O. Scrooge: Oh dear, are all females of the species this sensible?
[His tone is ambiguous, perhaps even patronizing.]
La Securité: Certainly: ... well, most of the time. However, we will
suspend judgement on your future, ... [Les Femmes glance
pointedly at the sharp stiletto heels on their shoes.]
... until after we have received our Christmas bonuses.
O. Scrooge: [He picks up a telephone.] Bob, is that you? Please
come back ... |
1. One (no longer used) method of obtaining hydrogen chloride involved the copper(II)-catalyzed reduction of chlorine;
(a) Complete the Table below using these bond energies (in kJ mol-¹): 463 (O-H), 243 (Cl-Cl), 432 (H-Cl), and 497 (O=O).
Bonds broken |
Energy absorbed / kJ mol-¹ |
Bonds formed |
Energy released / kJ mol-¹ |
Total = |
Total = | ||
[3]
Calculate the heat energy change (DH) for the above reaction. _________
_______________________________________________________________________
[2]
(b) Explain the purpose of the broken brick. __________________________
_______________________________________________________________________
[2]
(c) Complete and label this energy level diagram for the reaction.
Energy ___
___
_________________
_________________
___________________ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
___________________
_________________________________________________
Path of reaction
[5]
(d) Explain the effects, on the rate of this reaction and the yield of
hydrogen chloride, of using:
A high pressure _______________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
A high temperature ____________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
2. In industry, hydrogen chloride is usually obtained as a co-product
of the manufacture of chlorinated hydrocarbons; e.g.,(a) Suggest the rôle of light in this (substitution) reaction. ________
_______________________________________________________________________
[1]
(b) State briefly why the use of a high pressure in this reaction would
not increase the yield of products. ___________________________________
_______________________________________________________________________
[1]
(c) Suggest one advantage that this industrial method has over that
described in 1. _______________________________________________________
[1]
Dr. R. Peters Next Contents' List