METALS: IRON COMPOUNDS
Compounds of iron range in complexity from haemoglobin, whose structure
is similar to both chlorophyll-a and vitamin-B12, to the examples shown
in the reaction scheme.
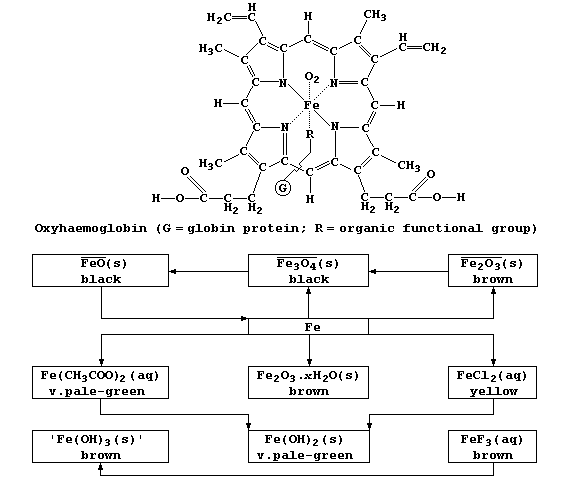
1. 1. Red blood cells contain the respiratory pigment haemoglobin, Hb.
In the absence of oxygen, a water molecule bonds to the iron(II) ion
to form deoxyhaemoglobin, HbH2O. Dioxygen reacts reversibly with HbH2O
to form oxyhaemoglobin, HbO2; this ligand-exchange reaction, which
involves an oxygen molecule replacing the water molecule and bonding
to the iron(II) ion, can be summarized in part by this equation:
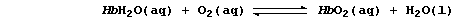
Carbon monoxide reacts reversibly with HbH2O, but irreversibly with
HbO2, to form carboxyhaemoglobin, HbCO; i.e.,
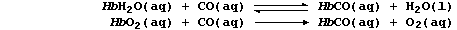
These three forms of haemoglobin colour the blood: dark-blue, HbH2O;
bright-red, HbO2; and cherry-red, HbCO. Cells which receive HbCO cannot
carry out aerobic respiration, and so cannot release sufficient ATP for
vital metabolic processes. Name two organs which contain cells that are
most likely to be affected by HbCO. ___________________________________
[2]
2. One method of preventing iron from forming rust, Fe2O3.xH2O(s), is
'to paint' the iron with a solution of phosphoric acid, H3PO4(aq); the
resulting layer of insoluble iron(III) phosphate provides the iron with
a physical barrier to water and dioxygen. Construct the symbol equation
for the reaction between iron and aqueous phosphoric acid. ____________
_______________________________________________________________________
3. Except for the precipitation reactions, leading to the formation of
iron(II) hydroxide and iron(III) hydroxide, explain carefully why all
the other reactions in the scheme are considered to be redox reactions.
_______________________________________________________________________
_______________________________________________________________________
[3]
4. Using a starting material shown in the scheme, construct the symbol
equation for one synthesis of:
Iron(II) ethanoate(aq) ________________________________________________
_______________________________________________________________________
Iron(III) fluoride(aq) ________________________________________________
_______________________________________________________________________
Iron(III) hydroxide(s) * ______________________________________________
_______________________________________________________________________
[6]
5. State and explain, using ionic equations, the observations at the
anode, at the cathode, and for the electrolyte when an aqueous solution
of iron(II) chloride is electrolyzed [the ions H1+(aq) and OH1-(aq),
though present, are not involved in the electrolysis].
Anode _________________________________________________________________
_______________________________________________________________________
Cathode _______________________________________________________________
_______________________________________________________________________
Electrolyte ___________________________________________________________
_______________________________________________________________________
[8]
6. Heterotrophs obtain their chemical energy via the hydrolysis of
organic compounds (e.g., carbohydrates, proteins, fats, and oils): by
contrast, autotrophs biosynthesize their chemical energy from inorganic
compounds (such as CO2, H2O, and H2S). The vast majority of autotrophs
are photosynthetic: but a significant minority are chemosynthetic. For
example, the bacteria Thiobacillus ferrooxidans obtain their source of
energy from the oxidation of iron(II) sulfide; the complex metabolic
processes involved can be loosely summarized by the following equation: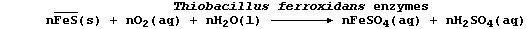
One of the metabolic by-products is dilute sulfuric acid. The passage
of this acid over ores containing insoluble copper(II) sulfide (CuS)
results in the formation of soluble copper(II) sulfate, which collects
in blue pools. Copper metal is extracted from these pools either by
addition of scrap iron or by electrolysis.
(a) Construct the symbol equation for the reaction between:
Aqueous sulfuric acid and copper(II) sulfide __________________________
_______________________________________________________________________
Iron and aqueous copper(II) sulfate ___________________________________
_______________________________________________________________________
[4]
(b) This extractive method for copper uses micro-organisms, albeit
indirectly, and so is an example of biotechnology; i.e., the industrial
use of genes from micro-organisms to obtain products considered useful
to Man. Genetic engineering, more correctly known as recombinant DNA
technology, maybe used to alter the genes of Thiobacillus ferrooxidans.
Suggest two possible applications for such altered bacteria. __________
_______________________________________________________________________
_______________________________________________________________________
[2]
* The currently accepted view is that the formula 'Fe(OH)3' is little
more than a convenient representation for various hydrated iron(III)
oxides; these substances are, incidentally, major constituents of soils
(and, as such, may be the commonest transition metal compounds observed
in everyday-life).
Dr. R. Peters Next Contents' List
