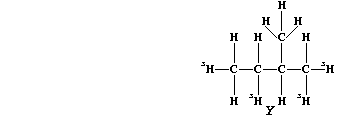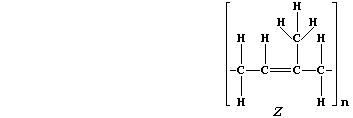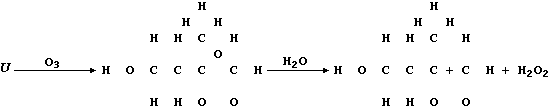ORGANIC CHEMISTRY: ISOPRENE
Many biologically important molecules, such as abscisic acid (a plant
growth regulator), cholesterol (a component of cell membranes), natural
rubber, oestrogen (a female sex hormone), retinal (a photochemical
pigment in the mammalian eye), and testosterone (a male sex hormone),
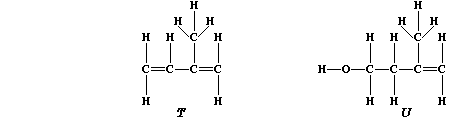
are based on a repeating five-carbon unit known as isoprene, "T", which
is biosynthesized from isopentenol, "U".
1. Suggest the reason why 'diene' is present in the systematic name of
isoprene (2-methylbuta-1,3-diene). ____________________________________
[1]
2. Manufacturers require far greater amounts of isoprene than can be
obtained from natural sources. Suggest how this synthetic intermediate
is obtained industrially in bulk quantities. __________________________
_______________________________________________________________________
[2]
3. Partial hydrogenation of isoprene gives two liquid alkenes, "V" and
"W": whereas complete hydrogenation gives liquid alkane "X".
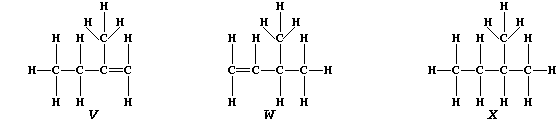
(a) Suggest one physical method, apart from chromatography, which could
distinguish between isomeric alkenes "V" and "W". _____________________
[1]
(b) Describe how you would show that a sample of "X" contained neither
"V" nor "W". __________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
(c) Saturation of diene "T" with the radioactive isotope of hydrogen-3
(tritium), gives labelled alkane "Y".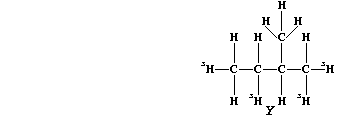
If this isotope of hydrogen (łH) has a half-life of 12.5 years, what
fraction of the labelled alkane "Y" would be present after 37.5 years?
_______________________________________________________________________
[2]
4. In a living organism, alcohol "U" is enzymically dehydrated to
diene "T". Name one catalyst which can speed up a similar dehydration
in the laboratory. ____________________________________________________
[1]
5. Natural rubber, "Z", is poly(isoprene). This hydrocarbon addition
polymer, which occurs as an aqueous suspension in the vascular system
of a number of plants, is usually extracted by 'tapping' rubber trees
(Hevea brasiliensis).
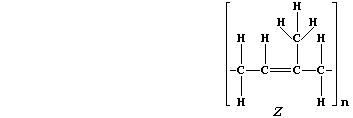
(a) Addition polymers and their corresponding monomers have identical
empirical formula, despite vastly different molecular formulae; e.g.,
poly(tetrafluoroethene), (-C2F4-)n, and tetrafluoroethene, C2F4, both
have the empirical formula CF2. What is the molecular formula of:
Natural rubber? ______________________ Isoprene? ______________________
[2]
(b) The molar mass of a monomeric unit in natural rubber is 68 g mol-ą.
If the molar mass of a sample of natural rubber is 1.02 x 10ł kg mol-ą,
calculate the value of 'n' in the molecular formula. __________________
_______________________________________________________________________
[1]
(c) Ozone, when produced by a complex series of photochemical reactions
involving nitrogen oxides and unburnt hydrocarbons emitted from vehicle
exhausts, is a component of photochemical smog. In sharp contrast to
its rôle in the upper atmosphere, protecting the biosphere from harmful
u.v. radiation, ozone causes the destruction of a diverse range of
synthetic and naturally occurring molecules. For example, the cracks
that appear in vehicle tyres often result from the corrosion of rubber
by ozone; such reactions can be summarized by this first scheme: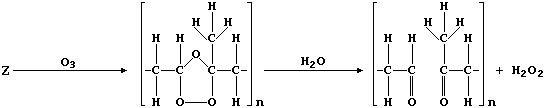
Complete the structural formulae shown in this second scheme, so as to
summarize the effect of ozone on the biosynthetic intermediate "U".
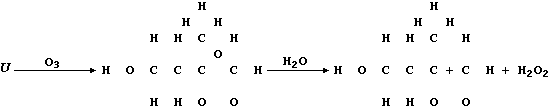
[3]
Suggest one type of naturally occurring molecule, which is involved in
growth or in reproduction, that might also be destroyed (or modified)
by the ozone present in smog. _________________________________________
[1]
(d) The major repositories for the biosphere's gene bank include the
rainforests: so their conservation is essential. The careful management
of rubber trees represents one renewable use of these resources. State
one non-renewable use of rainforests. _________________________________
_______________________________________________________________________
[1]
Dr. R. Peters Next Contents' List