METALS: MANGANESE
Manganese, the eighth most abundant metal in the Earth's crust (0.09%),
occurs mainly in the ore pyrolusite (impure MnO2). This transition
element, which has a high melting point (1244ฐC) and a high density
(7.44 g cm-ณ), forms compounds in several oxidation states [e.g.,
(pale-pink) manganese(II) sulfate, (red) manganese(III) sulfate,
(brown) manganese(IV) oxide, and (purple) potassium manganate(VII)].
[.. K > Ca > Na > Mg > Al > Mn > Zn > Fe > Sn > (H) > Cu > Hg > Ag ..]
1. Manganese is produced in two forms: ferromanganese, a carbon-
containing alloy used to manufacture hard 'manganese' steels; and the
pure metal, which is used to manufacture non-ferrous alloys (e.g.,
Duralumin and Manganin).
(a) Ferromanganese is formed when a mixture of haematite, pyrolusite,
coke, and limestone is heated in a furnace; the proportion of iron to
manganese in the alloy is dependent on the purity and ratio of the ores
in the mixture. The following symbol equation summarizes the conditions
used to obtain this alloy with a manganese content of 80%:
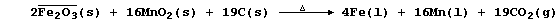
Suggest the formulae of two other substances formed in the furnace. ___
_______________________________________________________________________
[2]
(b) The pure metal, for which there is very little demand, used to be
obtained exclusively by the chemical reduction of purified pyrolusite: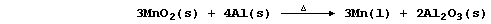
Construct the net ionic equation for the above redox reaction. ________
_______________________________________________________________________
Suggest one reason why aluminium is preferred to carbon as the reducing
agent. ________________________________________________________________
[3]
Partly because of the viciously exothermic nature of the above reaction
(DH = -1791 kJ mol-น), the currently preferred method of obtaining the
pure metal is by the electrolytic reduction of aqueous manganese(II)
sulfate; this salt is obtained by the oxidation of concentrated
sulfuric acid with manganese(IV) oxide: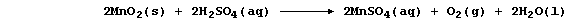
Explain, using a redox half equation, why the above equation summarizes
a redox reaction (and not a neutralization). __________________________
_______________________________________________________________________
[2]
2. Manganese(IV) oxide and potassium manganate(VII) are both used
extensively as oxidizing agents in laboratories and in industry.
(a) Although too expensive to be used on an industrial scale, the
standard laboratory preparation of chlorine gas involves the oxidation
of concentrated hydrochloric acid with manganese(IV) oxide. Construct
the symbol equation for this redox reaction. __________________________
_______________________________________________________________________
[2]
(b) Several companies use potassium manganate(VII) to purify water for
domestic use; aqueous manganate ions, MnO41-(aq), which can penetrate
cell walls and membranes, act as biocides by oxidizing a range of
essential biological molecules. This oxidizing agent is often preferred
to chlorine, for two reasons: one, it does not affect the taste of the
water; and two, the manganese(IV) oxide produced acts as a coagulating
agent for suspended particles. Write a redox half equation to represent
the change in oxidation state of manganese. ___________________________
_______________________________________________________________________
[1]
Name one other coagulating agent commonly used in water purification.
_______________________________________________________________________
[1]
3. Aqueous hydrogen peroxide decomposes slowly at ambient temperature;
the equation for this decomposition can be written as follows:
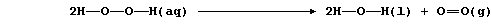
(a) Complete this Table using these bond energies (in kJ mol-น):
H-O (463); O-O (146); and O=O (497).
Bonds broken |
Energy absorbed
/ kJ mol-น |
Bonds formed |
Energy released
/ kJ mol-น |
|
|
|
|
|
|
|
|
Total = |
Total = |
[3]
Calculate the heat energy change (DH) for the above decomposition. ____
_______________________________________________________________________
[2]
(b) Catalase, an iron-containing enzyme with a structure very similar
to haemoglobin, and manganese(IV) oxide both speed up the decomposition
of hydrogen peroxide; though the enzyme is considerably more effective
than the inorganic catalyst, as might be expected. Complete and label
this energy level diagram for the decomposition of hydrogen peroxide
using these two different catalysts.
Energy ____
____
____
__________ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
__________
__________________
__________________
__________________________________________________
Path of reaction
[6]
4. Photosynthesis in plants can be summarized by this equation: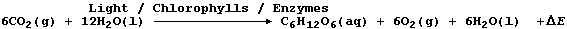
In one of the light-dependent reactions, a manganese-containing enzyme
is involved in the oxidation of water to form the oxygen gas;
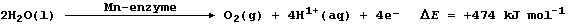
(a) Experiments have shown that water, and not carbon dioxide, is the
source of oxygen atoms in the oxygen gas formed. Using symbols, suggest
what 'type' of water was used in these experiments. ___________________
(b) Suggest the meaning of the term '+ DE'. ___________________________
_______________________________________________________________________
[2]
5. In mammals, urea is formed by the deamination of excess amino
acids; a manganese-containing enzyme, known as arginase, is involved in
this biochemical process.
(a) Name the other excretion product formed by the deamination of
excess amino acids. ___________________________________________________
(b) Where in a mammal is urea biosynthesized? _________________________
[2]
Dr. R. Peters Next Contents' List