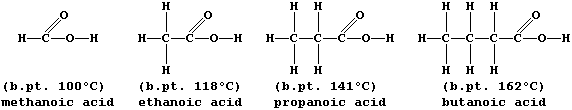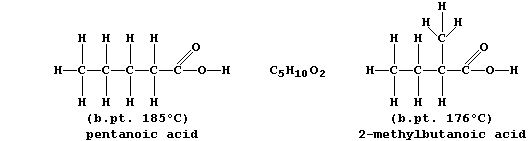ORGANIC CHEMISTRY: INTRODUCTION - DIVERSITY
Carbon has an unparalleled ability to form stable chains, rings, inter-
connected rings, and a wide range of single and multiple bonds (e.g.,
C=C, C=N, C=O, C=S, CºC, and CºN). Accordingly, despite carbon being
only the 17th most abundant element in the Earth's crust (0.09%), it is
not surprising that organic compounds are ubiquitous and so numerous.
Certainly, the extraordinary diversity of living organisms is directly
attributable to the facility of carbon atoms to form large and complex
structures, as exemplified by chlorophyll-a and adenosine triphosphate
(ATP) - two of the most important molecules in the biosphere. [See, in
particular, P. W. Atkins, Molecules, Scientific American Library, New
York, 1987; this book is absolutely stunning or, using the author's own
felicitous words, "... a book for occasional delectation."]
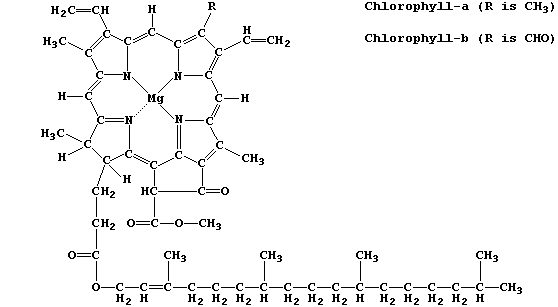
Chlorophyll-a is the commonest photosynthetic pigment; its function is
to absorb light energy and transduce it into chemical energy.
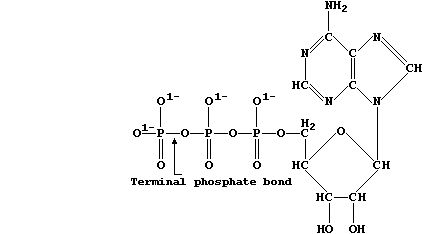
Adenosine triphosphate is the immediate source of chemical energy for
all living organisms; the energy required for the endergonic processes
in cells, such as active transport and biosynthesis, is released via
enzymic hydrolysis of ATP's terminal phosphate bond.
ORGANIC CHEMISTRY: INTRODUCTION - NOMENCLATURE
The systematic name of an organic compound is divided into two parts:
one part indicates the chain length, ring size, or type of ring, and
the other indicates the functional group(s). A functional group is an
atom or group of atoms bonded to the carbon skeleton, and its reactions
will often determine the chemical properties of the whole compound.
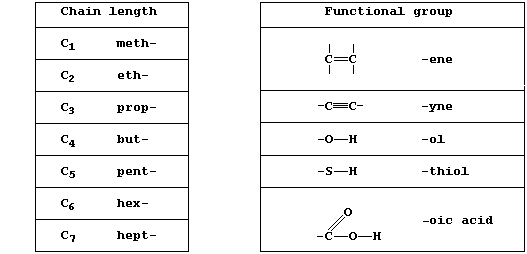
[Note, these two Tables present only a few of the prefixes and
suffixes used in the systematic naming of organic compounds. ]
A group of compounds are said to belong to the same homologous series
if they have the same functional group but different chain lengths or
ring sizes. Within a series, homologues usually show similar chemical
properties but different physical properties; e.g., all carboxylic
acids are neutralized by alkalis,
but their boiling points steadily increase with increasing molar mass:
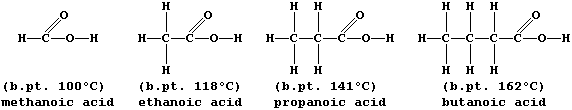
The molecular formulae of adjacent members of a series differ by CH2,
so each homologous series can be represented by a general formula (this
is CnH2nO2 for chain carboxylic acids). Finally, there are compounds
with the same molecular formula but different structural formulae -
these are known as isomers.
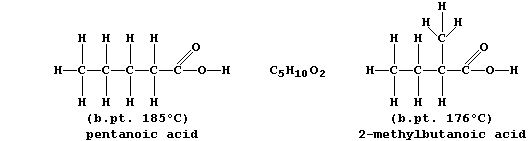
Dr. R. Peters Next Contents' List