ORGANIC CHEMISTRY: INTRODUCTION - PETROLEUM
Petroleum, which was formed over millions of years by the compaction
of dead organisms and the anaerobic decomposition of their biological
molecules, is usually found trapped beneath impermeable cap rock and
above a lower dome of sedimentary rock.
This oil, a non-renewable 'fossil fuel', is a mixture of solid, liquid,
and gaseous hydrocarbons (mostly alkanes). In refineries, crude oil is
fractionally distilled - a physical process which separates the various
components on the basis of their different boiling points:
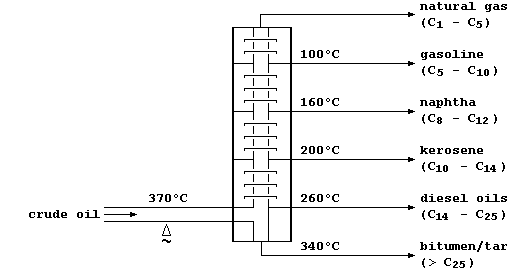
Some of these hydrocarbon fractions are used directly; e.g., as fuels
(natural gas), or to manufacture petrol (gasoline), or to surface roads
(tar). But, as this double bar-graph exemplifies, the demand for these
fractions rarely matches their relative amounts in typical crude oil.
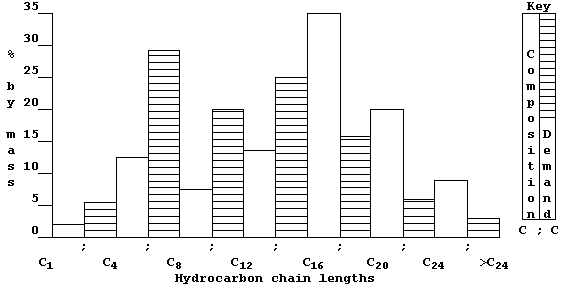
So, to overcome this economic problem, industry takes certain fractions
and subjects them to catalytic cracking - a chemical process whereby
large molecules are broken down by heat and by catalysis into smaller
(and more useful) molecules; e.g.,
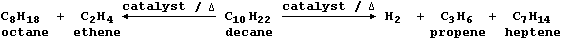
Mixtures of cracked products are separated by fractional distillation,
and the fractions used as fuels, solvents, or synthetic intermediates.
ORGANIC CHEMISTRY: INTRODUCTION - REACTION TYPES
The biological and industrial importance of organic compounds, together
with their structural diversity, has resulted in the development of
hundreds of synthetic methods. Happily, most of these can be classified
into just eight reaction types:
Addition (A), Condensation (C), Elimination (E), Hydrolysis (H),
Isomerization (I), Oxidation (O), Reduction (R), and Substitution (S).
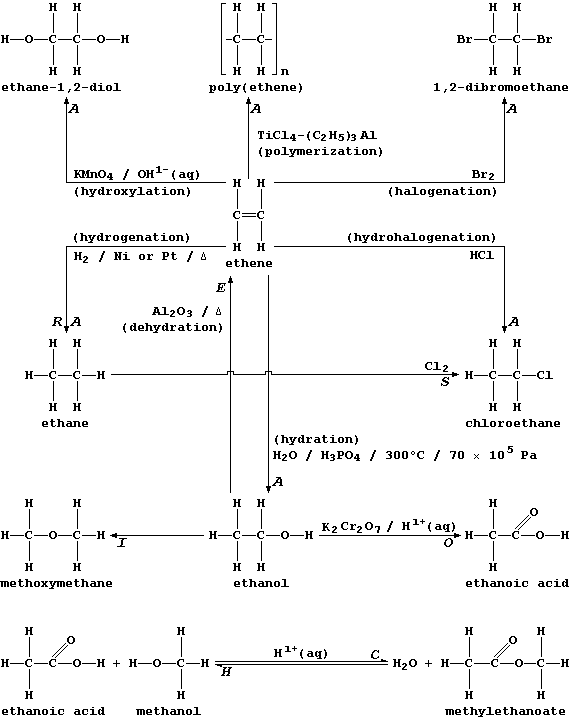
Two points are worth keeping in mind when considering the exemplars of
each reaction type shown above. First, more than one description can be
in common use for a given type; e.g., what is, formally, the addition
of hydrogen to ethene will also be described as the reduction or the
hydrogenation or the saturation of ethene. And second, each type can be
usefully viewed as one of a 'pair', as exemplified explicitly above by
addition-elimination and by condensation-hydrolysis.
Dr. R. Peters Next Contents' List


