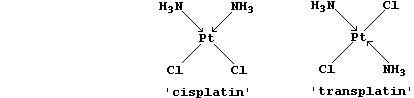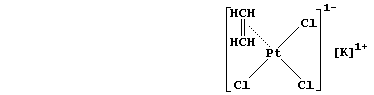METALS: PLATINUM
Platinum, one of the rarest elements in the Earth's crust (0.0000005%),
is usually found either native or as an alloy in various ores. This
element is a typical transition metal, as evinced by its high melting
point (1772°C), high density (21.45 g cm-³), variable oxidation states
[e.g., Pt(II) and Pt(IV)], and catalytic activity (e.g., Pt-Al2O3 is
used as a co-catalyst in the cracking of petroleum oil fractions).
[.... Zn > Fe > Co > Ni > (H) > Cu > Hg > Ag > Rh > Pd > Ir > Pt > Au]
1. There is no routine method for the extraction of platinum; the
processes used are highly dependent on the composition and quality of
the ores, which are invariably 'low-grade'. Currently, about 100 tonnes
of platinum are extracted each year. Calculate the mass of ore required
to obtain this mass of platinum, assuming that the platinum content of
the highest-grade ore is 0.000001%. ___________________________________
[1]
2. The central position of controlled experiments in science may give
the understandable impression that progress is achieved exclusively by
hypothesis testing (se non è vero, è ben trovato): but this is not the
case, either historically or contemporaneously. Thus, the discoveries
of the purple dye mauveine (1856), the antibiotic penicillin (1928),
the bactericide sulfonamide (1934), the sedative diazepam (1957), and
the carbon-allotrope buckminsterfullerene (1985) represent just five of
countless examples of serendipity; another is cisplatin, a platinum(II)
compound which unexpectedly showed anti-cancer activity in culture
cells (1968).
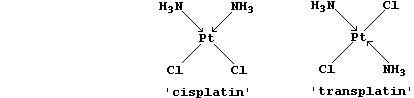
(a) Why are cisplatin and transplatin considered to be isomeric? ______
_______________________________________________________________________
[1]
(b) Following the serendipitous observation with cisplatin, scientists
synthesized and tested several thousand platinum(II) compounds in
attempts to obtain an anti-cancer drug with similar activity to, but
lower toxicity than, cisplatin (the so-called 'lead' compound). Draw
the structural formulae of two platinum(II) compounds which might show
similar chemical or biological properties to cisplatin. _______________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) Cancer, which is the uncontrolled mitotic division of cells, can
result from mutations (i.e., changes in the sequence of base pairs of
DNA). Accordingly, more recent studies have focused on the reactions
between platinum(II) compounds and the four nucleotide bases present in
DNA. Name these bases (usually represented by their initial letters:
'A', 'C', 'G', and 'T'). ______________________________________________
[4]
Mutations which occur in somatic cells may lead to cancer, but the
resulting genetic changes are not inheritable. By contrast, mutations
can be passed on to the offspring if they occur in the reproductive
cells of which two mammalian organs? __________________________________
[2]
3. Platinum's attractive appearance, high resistance to oxidation, and
high electrical conductivity has resulted in its use in jewellery and
as an electroplating metal. More importantly, despite (or because of)
its low chemical reactivity, platinum is used as a catalyst in at least
30 commercial processes. Indeed, the major current use of platinum is
as a co-catalyst in the 'catalytic convertors' of vehicle exhausts; one
of the many chemical reactions which occur in such convertors is:
Pt-Rh / D
2CºO(g) + 2NºO(g) —————————® O=C=O(g) + NºN(g)
(a) Complete this Table using these bond energies (in kJ mol-¹):
CºO (1069); NºO (627); C=O (745); and NºN (946).
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
|
|
|
|
|
|
|
|
Total = |
Total = |
[3]
Calculate the heat energy change (DH) for the above redox reaction. ___
_______________________________________________________________________
[2]
(b) Catalytic convertors undoubtedly reduce the emission of harmful
pollutants: but such benefits to the environment must be offset against
the potential destruction of ecosystems caused by extracting mineral
resources - particularly those of low natural abundance. Admittedly,
the precious metals are usually recycled when vehicles are scrapped or
convertors are rendered ineffective by 'catalyst poisoning': but, in
view of the increasing industrialization of the developing countries,
there must be doubts at to whether the use of precious metal catalysts
can be sustained. Consider, however, Vorsprung durch Biotechnik; the
lead compounds could be thermally robust enzymes isolated from species
of Archaebacteria (e.g., Methanococcus jannaschii), and recombinant DNA
technology could be used to alter the genes coding for these catalysts.
Suggest the meaning of the phrase 'thermally robust enzyme'. __________
_______________________________________________________________________
[1]
4. Finally, un divertissement ... The first organometallic compound
isolated was Zeise's salt (1825). Shown below is the structural formula
of this platinum(II)-ethene compound; the [PtCl3(C2H4)]1- ion is square
planar, with chloride at three corners and ethene at the other corner -
however, the ethene molecule is perpendicular to the PtCl3.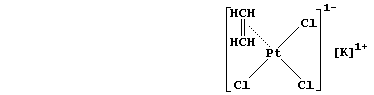
Zeise's salt has no commercial use, but related palladium(II)-alkene
compounds are catalysts in various industrial processes. Draw the
structural formula of [PdCl3(C2H4)]1-Na1+ and of trans-PdCl2(C2H4)2.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
Dr. R. Peters Contents' List