SELECTED PRINCIPLES: RATES OF REACTIONS (1)
During any chemical reaction the concentrations of reactants decrease
and the concentrations of products increase. The rate of reaction is
the change in the concentration of reactants or products with time;
and this change can be determined by measuring any convenient chemical
or physical property of the reaction mixture (e.g., colour intensity,
electrical conductivity, mass, pH, precipitate formation, temperature,
or volume of gas).
Independent variables known to affect the rates of reactions include
concentration, pressure, surface area, the presence of an inhibitor,
temperature, light, and the presence of a catalyst. The effect of these
particular variables on reaction rates can be explained qualitatively
by the use of collision theory; i.e., particles must collide before
they react, and that a reaction will only occur if the colliding
particles contain more than a certain minimum amount of energy - known
as the activation energy. Thus, ...
An increase in concentration, or in pressure (for gases), increases the
number of collisions between the particles.
An increase in the surface area of reactants, which are either solids
or immiscible liquids, increases the number of particles exposed to
collisions.
An inhibitor decreases the number of collisions between the particles,
effectively by reducing either the concentration or the surface area
of reactants or catalyst.
An increase in temperature results in an increase in the kinetic energy
of the particles, so more will have the required activation energy for
successful collisions.
Light energy absorbed by reactants is transduced into chemical energy,
so more of these particles will have the required activation energy for
successful collisions.
A catalyst decreases the activation energy of a reaction, so more
particles will have the required energy for successful collisions.
Quantitative investigations measuring rates of reactions follow the
standard precepts of controlled experiments ... First, a hypothesis is
formulated, which states a predicted relationship between a dependent
variable and one independent variable. Second, values of the dependent
variable are measured as values of the chosen independent variable are
changed systematically, whilst all other variables are both measured
and held constant (so as 'to isolate the independent variable'). And
third, the data are examined critically to determine whether there is
evidence to support the formulated hypothesis; this examination must
include an assessment of the various sources of experimental error.
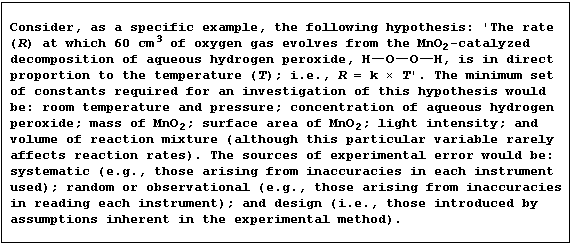
1. The equation for the decomposition of aqueous hydrogen peroxide is:
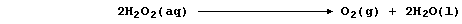
This first sketch graph shows the effect of changing light intensity on
the rate of decomposition of aqueous hydrogen peroxide.
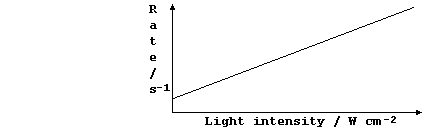
These four sketch graphs show the effects, on the rate of decomposition
of aqueous hydrogen peroxide, of the following independent variables:
concentration of a soluble inhibitor; volume of reaction mixture; type
of catalyst; and, temperature of reaction mixture in the presence of
the enzyme catalase. Label each graph with the correct independent
variable and a suitable unit.
2. In order to confirm the catalytic activity of a solid substance,
what two measurements of the substance need to be made (apart from
measuring increases in reaction rate)? ________________________________
_______________________________________________________________________
[2]
3. What physical property of a solvent usually determines the minimum
temperature for a reaction carried out in solution? ___________________
[1]
4. As a 'rule of thumb', the rate of a chemical reaction doubles for
every 10°C increase in temperature. If the rate of a certain chemical
reaction was 32 s-¹ at 40°C, what would be its rate at 0°C? __________
_______________________________________________________________________
_______________________________________________________________________
[2]
5. Name two refrigerants used to preserve biological materials such
as reproductive cells. ________________________________________________
[2]
6. Visible light is a region of the electromagnetic spectrum (e.m.s).
Name two other regions of the e.m.s. which might increase the rate of
certain chemical reactions. ___________________________________________
[2]
Dr. R. Peters Next Contents' List

