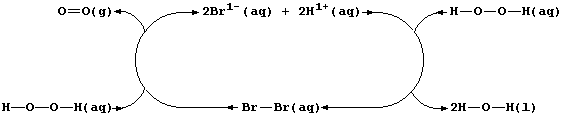SELECTED PRINCIPLES: RATES OF REACTIONS (2)
Until relatively recently, catalysts had acquired the status of being
merely 'wizard's potions', with a particular potion increasing the rate
of a certain chemical reaction by lowering its activation energy.
However, motivated partly by the recognition that 'Nature has designed'
enzymes which are more efficient than the catalysts discovered by Man,
and partly by industry's demands for more efficient catalysts (so as to
reduce energy costs), researchers have begun to unravel the details of
catalysis. Most unfortunately, even a superficial understanding of this
research requires a fairly advanced knowledge of atomic structure and
chemical bonding; on the other hand, even a 'bird's-eye-view' of one
reaction can provide a useful point of reference ...
Aqueous dibromine is but one of several substances known to catalyze
the decomposition of aqueous hydrogen peroxide; i.e.,
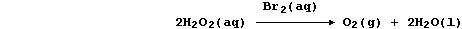
The experimental evidence is consistent with the interpretation that
this reaction occurs essentially in two steps. In the first, dibromine
oxidizes hydrogen peroxide to aqueous hydrogen ions and dioxygen; i.e.,
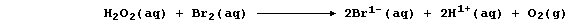
Then, in the second, the bromide ions are oxidized back to dibromine by
more hydrogen peroxide; i.e.,
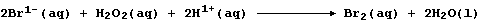
Thus, dibromine reacts in the first step and is then regenerated in the
second; so, the substance is not used up in the reaction.
Each catalyst increases the rate of a reaction without being chemically
changed at the end of a reaction. Accordingly, each reaction involving
a true catalyst can always be represented by a closed loop; the various
catalytic species form the main body of the loop, and the reactants and
products enter and leave the loop at various places.
Shown below is a closed loop for the dibromine-catalyzed decomposition
of aqueous hydrogen peroxide.
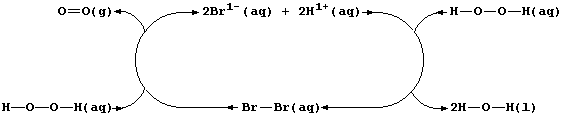

1. Nitrogen-fixation is the term used to describe the conversion of
molecular dinitrogen into compounds of nitrogen.
(a) Industrial fixation involves the reduction of dinitrogen to ammonia
using an iron catalyst, high temperatures, and high pressures; e.g.,
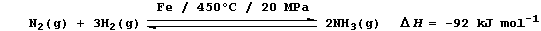
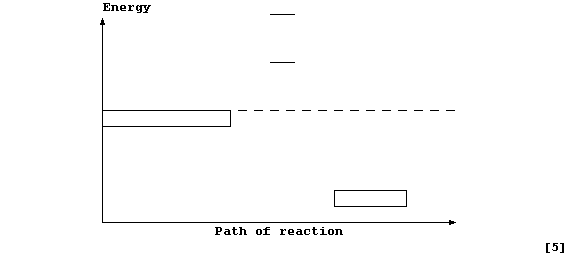
Complete and label this energy level diagram for the above reaction.
By reference to the Periodic Table, name two elements which might show
similar catalytic activity to iron. ___________________________________
[2]
One mole of any gas occupies a volume of 24000 cm³ at room temperature
(25°C) and pressure (0.1 MPa). Calculate the density of ammonia at
r.t.p. ________________________________________________________________
_______________________________________________________________________
[2]
(b) Biological fixation involves the Mo-containing enzyme nitrogenase,
under ambient conditions, in an ostensibly similar reduction; one of
the important reactions can be loosely summarized by this equation: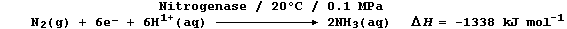
Suggest and explain the reason why this reaction is considerably more
exothermic than that which occurs in industrial fixation. _____________
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) Atmospheric fixation results in the formation of various nitrogen
oxides. Name two sources of energy involved in this type of nitrogen-
fixation. _____________________________________________________________
[2]
2. Carbon-fixation is the term used to describe the incorporation of
carbon dioxide into organic compounds; as occurs, for example, during
the biological process of photosynthesis: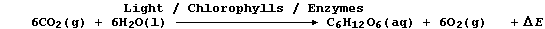
The kingdom Monera consists of three subkingdoms: Eubacteria (formerly
Bacteria; e.g., Bacillus anthracis, Clostridium botulinum, Escherichia
coli, Treponema pallidum, and Vibrio cholerae); Cyanobacteria (formerly
Blue-green Algae; e.g., Nostoc commune); and Archaebacteria (e.g.,
Methanococcus jannaschii). The most nutritionally independent organisms
in the biosphere are species of Cyanobacteria which can carry out both
nitrogen-fixation and carbon-fixation (via photosynthesis). State what
these organisms require for survival, apart from a suitable temperature
and sunlight. _________________________________________________________
[4]
Dr. R. Peters Next Contents' List