METALS: TIN & LEAD
Tin, a rare element in the Earth's crust (0.0002%), occurs mainly in
the ore cassiterite [impure tin(IV) oxide]. The metal was obtained
first in the Bronze Age, when a mixture of coal and rocks was heated
in air; so, presumably, one of the reactions that occurred was:
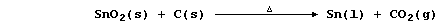
Modern technology uses coke and purified cassiterite in a furnace: but,
otherwise, the extractive method used to obtain tin, as well as copper
and lead, has not fundamentally changed over 7000 years.
[.. K > Ca > Mg > Al > Zn > Fe > Co > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. The principal ore of lead, a relatively rare element (0.001%) in
Earth's crust, is galena [impure lead(II) sulfide]. Lead(II) oxide is
formed when this ore is heated in air, and the metal is extracted by
the chemical reduction of this oxide with carbon in a furnace.
(a) Construct the symbol equation for each reaction in this process.
_______________________________________________________________________
_______________________________________________________________________
[4]
(b) Carbon and carbon monoxide are the commonest reducing agents used
industrially to reduce metal oxides, but others are also used (e.g.,
aluminium and dihydrogen). In the laboratory, reductions with hydrogen
gas can be attempted using the apparatus shown in this diagram.
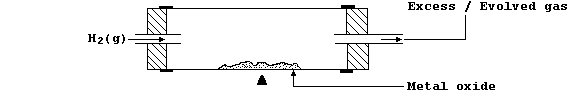
This is an open system in which equilibrium is never reached; thus, as
predicted by Le Chatelier's Principle, the stream of dihydrogen ensures
that the position of equilibrium continually 'shifts to the right'.
The Table below shows the calculated heat energy changes for the
reduction of various metal(II) oxides with dihydrogen; i.e.,
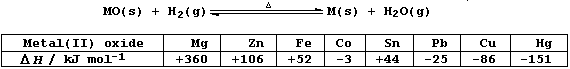
State and explain the effect of a temperature increase on the:
Rate of each reaction _________________________________________________
_______________________________________________________________________
_______________________________________________________________________
Yields of products in each endothermic reaction _______________________
_______________________________________________________________________
_______________________________________________________________________
Yields of products in each exothermic reaction ________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
2. Tin, (m.pt. = 232°C; r = 7.29 g cm-³), and lead, (m.pt. = 327°C; r
= 11.34 g cm-³), are both used extensively in alloys. Name two alloys
which contain either or both metals. __________________________________
[2]
3. Tin is used to manufacture 'tin-plate' (i.e., steel plated with a
layer of tin). Explain how tin protects steel from corrosion. _________
_______________________________________________________________________
_______________________________________________________________________
[2]
4. Approximately half of the lead produced is used in the manufacture
of storage batteries; in turn, these are recycled to provide about 40%
of industry's requirements of lead. A lead storage battery consists of
six cells connected in series; each 2 V cell, which has a lead anode, a
cathode made of lead(IV) oxide, and an electrolyte of aqueous sulfuric
acid, delivers high currents for short periods of time.
(a) When a battery discharges (i.e., when in use providing the required
voltage), the reactions occurring at the electrodes are:
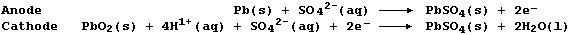
State the energy change which occurs during discharge. ________________
_______________________________________________________________________
[2]
(b) Suggest and explain one reason why such batteries are labelled with
the hazard symbol 'No smoking'. _______________________________________
_______________________________________________________________________
[2]
(c) Recharging the battery involves applying an external voltage to the
anode and cathode. What name is given to this reversal of the above
electrochemical reactions? ____________________________________________
[1]
5. Compounds of tin and lead have few (if any) beneficial functions in
living organisms. However, lead compounds are known to be particularly
toxic, because lead(II) ions inhibit the 'active sites' of a number of
enzymes; furthermore, as with so many non-biodegradable biocides, they
accumulate up the trophic levels.
(a) Name two sources of the lead ions introduced into the environment,
apart from lead storage batteries and mine-workings. __________________
_______________________________________________________________________
[2]
(b) The diagram below represents one food chain in the complex food web
of a typical slow-moving river in a temperate region.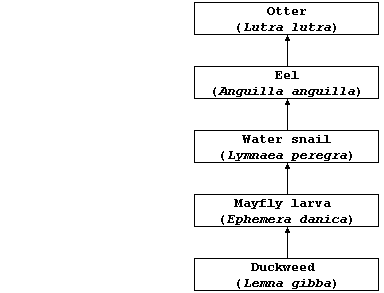
Estimate the amount of lead(II) ions which could be accumulated by one
otter, assuming: that each consumer in this food chain obtains its
chemical energy by eating 10 individuals of the organism at the trophic
level immediately below it; that none of the consumers egest or excrete
these ions; and that each duckweed plant absorbed 0.01 mg of these
ions. _________________________________________________________________
[2]
Name the source of energy for the producer in this food chain. ________
[1]
Dr. R. Peters Next Contents' List


