REFERENCE SECTION: FORMULAE and SOLUBILITIES of SALTS
Salts are defined as 'ionic compounds which contain cations other than
hydrogen and anions other than oxide or hydroxide'. However, salts may
be more usefully considered as ionic compounds in which the ionizable
hydrogen ions of an acid have been replaced by either metal or ammonium
ions. A monoprotic acid, such as ethanoic acid, has one ionizable
hydrogen, and so can only form a neutral salt: by contrast, a diprotic
acid, such as carbonic acid, has two ionizable hydrogens, and so forms
both neutral and acid salts. In general, neutral salts are less soluble
in water but more thermally stable than their acid analogues; compare,
for examples, sodium carbonate with sodium hydrogencarbonate or calcium
carbonate with calcium hydrogencarbonate.
Complete the Table below, which summarizes the solubilities in water
and the formulae of typical neutral salts (formally) derived from five
acids [HCl(aq) ; H2SO4(aq) ; CH3COOH(aq) ; H2CO3(aq) ; and HNO3(aq)],
by inserting the correct formula for each salt. * # §
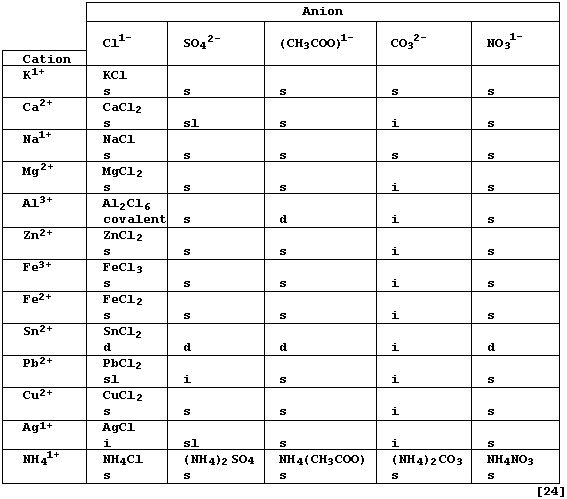
* Each formula refers to the anhydrous salt, although many of these
compounds crystallize from aqueous solutions as hydrates; e.g., calcium
chloride-water (1/6). The anhydrous salt can sometimes be obtained from
from the hydrated salt either by the use of certain dehydrating agents
(e.g., concentrated sulfuric acid), or by careful heating (though this
is often accompanied by thermal decomposition of the anhydrous salt).
# Solubility in water at 20°C: d = decomposes (i.e., hydrolyzes); i =
insoluble (< 1 g kg-¹); sl = slightly soluble (1 - 10 g kg-¹); and s =
soluble (> 10 g kg-¹).
§ Ionic compounds, which tend to have high melting points, conduct
electricity when their ions are free to move (i.e., in the molten state
or when dissolved in water: but not in the solid state).
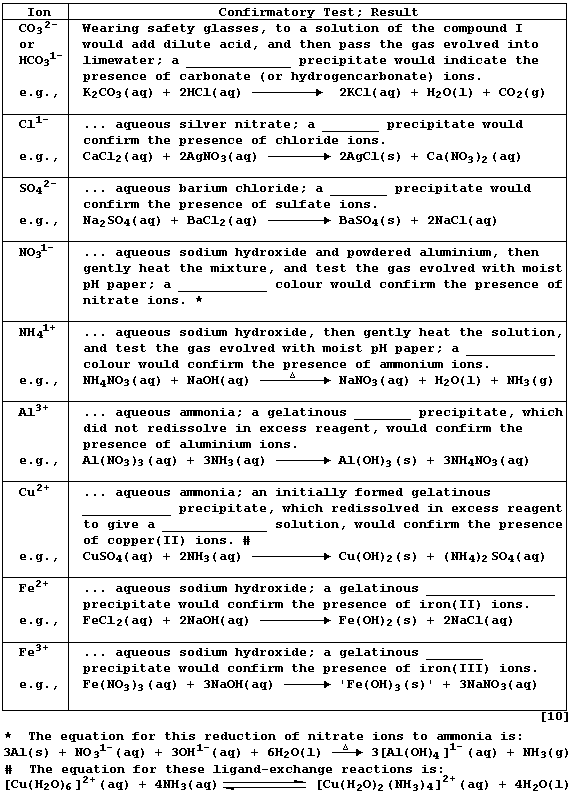
REFERENCE SECTION: TESTS for ANIONS and CATIONS
Confirmatory tests for ions share two common features. First, each test
is executed on a solution of the compound; this is usually prepared by
dissolving the solid in either distilled water or dilute nitric acid.
And second, reaction of an aqueous solution of the ion with the test
reagent results in either the selective precipitation of the ion or the
selective evolution of a characteristic gas.
Complete the Table below, which summarizes tests for ten common ions,
by inserting the correct observation from this list: brown; dark-blue;
milky-white; pale-blue; royal-blue; very pale-green; white.
Dr. R. Peters Next Contents' List

