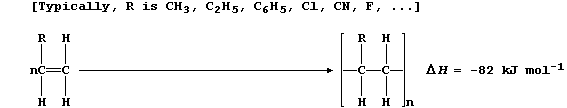METALS: TITANIUM
Titanium, the seventh most abundant metal in the Earth's crust (0.6%),
is usually found as an oxide (e.g., in the ores rutile and ilmenite).
This transition element, which has a high melting point (1660°C) but
only a moderately-high density (4.51 g cm-³), shows variable oxidation
states [e.g., (coloured) Ti(III) and (colourless) Ti(IV)].
[.. K > Ca > Na > Mg > Al > Ti > Zn > Fe > Pb > (H) > Cu > Hg > Ag ..]
1. The extraction of titanium from its principal ores is complex, but
the final process involves chemical reduction of titanium(IV) chloride
with magnesium at 1050°C in an atmosphere of argon.
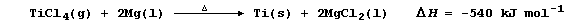
(a) Titanium metal is expensive, because its extraction involves high
costs in terms of energy, raw materials, and ensuring safety. From a
consideration of the above equation, suggest and explain one possible
way of reducing energy costs. _________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Suggest one reason why it is necessary to carry out this reduction in
an atmosphere of argon. _______________________________________________
_______________________________________________________________________
[1]
(b) The covalent compound titanium(IV) chloride, a liquid at room
temperature, rapidly hydrolyzes to give an acidic solution and a white
solid (Mr = 80). Construct the symbol equation for this hydrolysis. ___
_______________________________________________________________________
[2]
2. Titanium, despite its high position in the reactivity series, does
not react with water, steam, or cold dilute acids; this observed lack
of reaction is due to the presence of a protective oxide layer. Explain
why this oxide layer reduces titanium's rate of reaction with several
reactants to effectively zero. ________________________________________
_______________________________________________________________________
[2]
3. There is a demand for alloys which incorporate both titanium and
aluminium. Suggest two properties of such alloys. _____________________
_______________________________________________________________________
[2]
4. During the past few decades, manufacturers have used alternatives
to lead compounds as pigments in paint; in particular, there is now
extensive use of titanium(IV) oxide - which is very stable, non-toxic,
and brilliant white. Perhaps surprisingly, this titanium pigment is not
usually obtained from purified rutile (TiO2), but from ilmenite.
(a) Analysis showed that a 5.00 g sample of pure ilmenite had this
composition by mass: iron, 1.86 g; titanium, 1.56 g; and oxygen as the
only other element present.
|
Fe |
Ti |
O |
Mass combining (m) / g |
|
|
|
Molar mass (M) / g mol-¹ |
|
|
|
Number of moles combining (m ÷ M)
|
|
|
|
Simplest ratio of number of moles |
|
|
|
Complete the above Table, so as to determine the empirical formula of
ilmenite. _____________________________________________________________
[5]
(b) Suggest one reason why there has been a decrease in the use of lead
compounds in the manufacture of paints (and petrol). __________________
_______________________________________________________________________
[1]
5. A characteristic reaction of alkenes is addition polymerization.
One of the many versatile reaction schemes used to synthesize addition
polymers is summarized below:
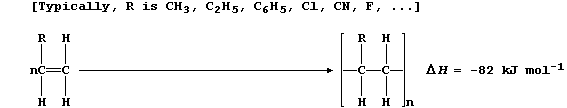
The general formula given above for an addition polymer is, however,
a shade misleading because analyses have shown that most addition
polymers can be obtained in at least three different forms: i.e.,
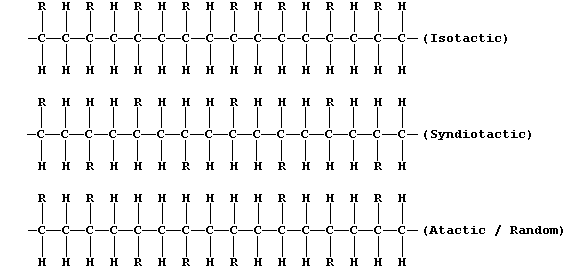
(a) What name is given to compounds which have the same molecular
formula but different structural formulae? ____________________________
[1]
(b) Suggest two physical methods which could be used to distinguish
between the different forms of an addition polymer (containing the same
functional group R). __________________________________________________
[2]
(c) Several transition metal compounds are known to act as catalysts
for the polymerization of alkenes; their use usually results in higher
yields of the isotactic and syndiotactic forms of addition polymers.
Titanium(IV) chloride is often stated to be a polymerization catalyst:
however, experiments have shown that the true catalyst is titanium(III)
chloride [which is formed by adding triethylaluminium, (C2H5)3Al, to a
reaction mixture of titanium(IV) chloride and alkene]. State the two
measurements that are required to determine whether a substance is
acting as a catalyst (apart from a comparison of reaction rates with
and without the substance). ___________________________________________
_______________________________________________________________________
[2]
6. Naturally occurring titanium contains five isotopes; the three most
abundant are Ti-46 (8.0%), Ti-47 (7.3%), and Ti-48 (73.8%). The most
stable radioactive isotope, titanium-44, has a half-life of 47 years.
Calculate the exact relative atomic mass of a sample of titanium which
contains only the three most abundant isotopes. _______________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
Dr. R. Peters Next Contents' List