METALS: EXTRACTION of ZINC
Zinc, which is relatively rare in the Earth's crust (0.007%), occurs
mainly as the sulfide or the carbonate (e.g., in the ores zinc blende
and calamine, respectively). This element is not considered to be a
transition metal, despite its position on the Periodic Table, partly
because it forms compounds which are (usually) white or colourless and
in only one oxidation state (II).
[.. K > Ca > Na > Mg > Al > Zn > Cd > Fe > Sn > Pb > (H) > Hg > Cu ..]
1. Extracting natural resources invariably produces a conflict between
economic advantages and conservation of the environment. For example,
open-cast mining of zinc ores usually provides employment for the local
population: but also produces large quantities of waste rock (known as
spoil), as well as the disruption or destruction of delicately balanced
ecosystems. Suggest one conservation measure which should be undertaken
when resources are extracted. _________________________________________
_______________________________________________________________________
[1]
2. Zinc is extracted and purified by the processes summarized in this
flow diagram.

(a) Combustion of crude zinc blende usually results in a mixture of
metal and silicon oxides, because the sand-bearing ores of zinc, lead,
and cadmium often occur together. Construct the symbol equation for the
complete combustion of (pure) zinc sulfide. ___________________________
_______________________________________________________________________
[2]
State the most important use of the by-product (i.e., sulfur dioxide).
_______________________________________________________________________
[1]
(b) Construct the symbol equation for the neutralization reaction of
zinc carbonate and dilute sulfuric acid. _____________________________
_______________________________________________________________________
[2]
(c) In the electrolytic reduction of aqueous zinc sulfate, which
contains the ions Zn2+(aq), SO42-(aq), H1+(aq), and OH1-(aq), dioxygen
is evolved at the carbon-graphite anode and zinc is deposited at the
carbon-graphite cathode. Write an ionic equation for the reaction which
occurs at the:
Anode _________________________________________________________________
Cathode _______________________________________________________________
[3]
Suggest and explain one reason why zinc is not extracted by the
electrolytic reduction of zinc oxide. _________________________________
_______________________________________________________________________
[2]
(d) Impure zinc is obtained when a mixture of oxides, obtained from the
combustion of crude zinc blende, is heated with coke and limestone in a
furnace at a temperature of about 1000°C. Fairly pure zinc, separated
from impurities by physical methods (e.g., fractional distillation), is
then either used directly or further purified electrolytically.
Coke, the source of chemical energy in the blast furnace, is burnt both
to release heat energy and to provide the main reducing agent:

Calcium oxide, formed by thermal decomposition of limestone, reacts
with silicon oxide to form slag (which is less dense than molten lead):
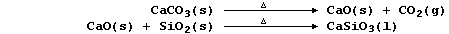
|
Zinc |
Cadmium |
Lead |
Melting point / °C |
420 |
321 |
328 |
Boiling point / °C |
908 |
765 |
1751 |
Density (r) / g cm-³ |
7.14 |
8.64 |
11.34 |
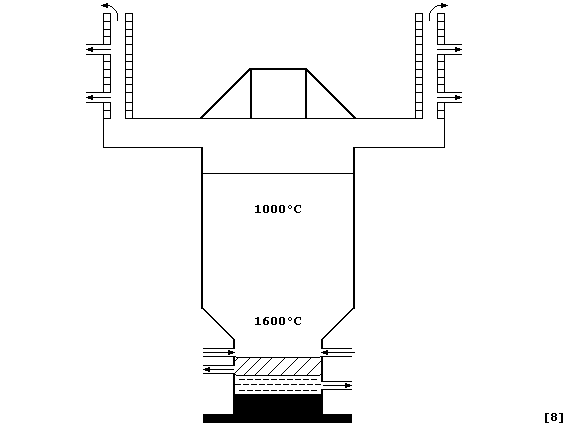
Noting the properties given in the Table, label this diagram of a zinc
blast furnace with: CaSiO3(l); Cd(l); Condenser; Hot air blast; Pb(l);
Reactants (oxides, coke, and limestone); Waste gases; and, Zn(l).
Construct an explanation, complete with two symbol equations, why
calamine could be used directly as a raw material in the blast furnace.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
What two physical processes are involved in fractional distillation?
_______________________________________________________________________
[2]
3. Zinc is used in alloys, in batteries, and to galvanize iron. If the
trends in present-day use of zinc continues, it has been guesstimated
that the known reserves of zinc ores will last for perhaps no more than
20 years. Suggest two ways of overcoming this potential shortage of
zinc. _________________________________________________________________
_______________________________________________________________________
[2]
Dr. R. Peters Next Contents' List
