AN INTRODUCTION TO CHEMICAL EQUATIONS
Chemical equations represent an essential part of the common language of scientists. Accordingly, one needs to acquire the skills in both their construction and interpretation. Setting aside the important fact that all equations are derived from experiments, their construction 'simply' requires practice and patience using, wherever possible, model examples: so, the text below focuses on the provision of a sound basis
for their interpretation.
A Chemical Equation
In a chemical reaction, the starting materials are known as reactants and the substances formed as products. A chemical equation is used to partially summarize a reaction. Thus, using chemical symbols, reactants are placed on the left and products on the right of an arrow; and, to conform with the Law of the Conservation of Mass, there must be the same number of each type of atom on both sides of the equation; e.g.,
![]()
Equation 1, which summarizes the combustion of carbon monoxide, has two
equivalent meanings: first, 'two molecules of gaseous carbon monoxide and one molecule of gaseous dioxygen react to give two molecules of gaseous carbon dioxide'; and second, 'two moles of gaseous carbon monoxide and one mole of gaseous dioxygen react to give two moles of gaseous carbon dioxide'.
Equation 1 is absolutely complete, in the sense that nothing needs to be added. Nevertheless, in attempts to provide more complete summaries of reactions, scientists often festoon equations with a wide variety of additional information; furthermore, equations are often replaced by reaction schemes ...
State Symbols
Such symbols are abbreviations for the physical states of the reactants and products; (aq), (g), (l), and (s) denote 'dissolved in water', gas, liquid, and solid, respectively. In the absence of state symbols, the reactants and products are assumed to be in their usual physical states at room temperature (288 - 298 K) and pressure (100 kPa).
Equation 2 is merely equation 1 modified to include state symbols; and,
in this particular example, cannot be regarded as a useful improvement.
![]()
However, the absence of state symbols can result in errors. *
Equation 3 has adorned many textbooks, examination papers, and scripts.
![]()
The precise interpretation of Equation 3 is, 'one mole of gaseous hydrogen chloride and one mole of solid sodium hydroxide react to give one mole of solid sodium chloride and one mole of liquid water': but, under rigorously anhydrous conditions, such a chemical reaction would be unlikely to occur; i.e.,
![]()
Equation 3 is frequently used to summarise the neutralization reaction between aqueous solutions of hydrochloric acid and sodium hydroxide: but the correct equation for this reaction is as follows.
![]()
* The following anecdote may be illuminating. If there is one 'fact' that people of un certain âge remember, from their best-forgotten days studying Chemistry, it is that copper(II) sulfate is blue ... An aqueous solution of copper(II) sulfate, CuSO4(aq), is indeed blue; crystals of hydrated copper(II) sulfate, CuSO4.5H2O(s), are also blue: however, copper(II) sulfate, CuSO4(s), is off-white.
Energy Changes
Each chemical reaction is accompanied by the formation of one or more new substances and an energy change (DE). Various symbols are used to provide a qualitative (or quantitative) indication of such a change in
either a single reaction or a process, in particular:
- DE, exergonic (i.e., energy is released to the surroundings);
+ DE, endergonic (i.e., energy is absorbed from the surroundings);
- DH, exothermic (i.e., heat energy is released to the surroundings);
+ DH, endothermic (i.e., heat energy is absorbed from the surroundings).
[In more advanced studies, the commonest symbol used to indicate the energy change of a reaction is DG (i.e., the change in 'free energy').]
Reaction Conditions
Most chemical reactions are carried out under ambient conditions; i.e., under laboratory lighting at room temperature and pressure. Information about reaction conditions, other than ambient, is placed on top of the arrow; e.g., named catalyst, light, heat, low or high temperature, and low or high pressure.
Reversible Reactions
In principle, each chemical reaction is reversible; i.e., it can occur in both directions (and can be made to proceed in either direction by altering the conditions): and so each equation should contain a double-headed arrow. In practice, however, conditions are chosen which usually ensure that there is complete conversion of reactant(s) to products(s); and if this has been shown (or assumed) to be so, then such a reaction is considered to be irreversible and is correctly summarized by an equation which contains a single-headed arrow.
In industry, it is not always feasible to use conditions which ensure irreversibility; thus, primarily for reasons of safety and economics, many industrial-scale syntheses involve reversible reactions; e.g., ...
Nitrogen monoxide, an intermediate in the manufacture of nitric acid, is obtained by the catalytic oxidation of ammonia; the symbol equation for the industrial-scale synthesis of this gas is:
![]()
The meaning of this equation is as follows: 'at a temperature of 850°C and a pressure of 200 kPa, in the presence of a platinum-rhodium co-catalyst, the reaction of gaseous ammonia and dioxygen is reversible (with incomplete conversion to gaseous nitrogen monoxide and water); in
a closed system, the forward and reverse reaction rates will be equal at dynamic equilibrium'.
Reaction Schemes
A chemical equation is, strictly speaking, the only correct method of summarizing a reaction. Nevertheless, in the scientific literature, the appearances of such equations are fairly rare. One reason for their rarity is that many symbol equations are quite complicated; e.g., the oxidation of ethanol to ethanoic acid by acidified dichromate(VI) is:
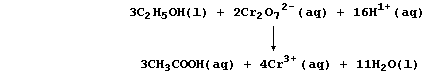
So, rather than bespatter their text with such 'monstrosities', authors prefer to use a reaction scheme. In this 'chemical shorthand', only the reactant and product of interest are stated explicitly; the reagent(s) and conditions are placed over the arrow. So, the oxidation of ethanol to ethanoic acid, for example, might be summarized by this scheme.
![]()
Perdre la grâce (ou Tomber en disgrâce)
There are two pivotal moments in the development of a toddler: first, when he or she realizes that 'Postie' probably does not hop on a sleigh to the North Pole; and second, when he or she decides to collude with adults in perpetuating the polite fiction of Father Christmas. Perish the thought that examples of such fiction occur in Chemistry, but note carefully the following sentence (J. Barrett, Understanding Inorganic Chemistry, Ellis Horwood, Chichester, 1991): "At the school level, some earlier theories are put forward as present-day explanations."
Introductory courses invariably include a suite of experiments designed to illuminate the important differences between elements, mixtures, and compounds. By long-established tradition, the commonest suite involves the following substances: the elements iron and sulfur; a mixture of iron and sulfur; and the 'compound' iron(II) sulfide ...
A dark-gray solid is formed when a mixture of the above two elements is gently heated; hitherto, this (fairly vigorous exothermic) process has been summarized by the following equation.
![]()
This dark-gray solid, usually designated as the stoichiometric compound iron(II) sulfide (FeS), has different chemical properties to both the elements and the mixture; e.g., dilute hydrochloric acid reacts slowly with iron to give the gas dihydrogen,
![]()
whereas the same acid reacts rapidly with the dark-gray solid to give the (toxic) gas hydrogen sulfide,
![]()
[Incidentally, this latter reaction is a splendid counterbalance to the oft-stated paradigm 'acid + base gives salt + water only'.]
Chemists had known for decades that the designation of this dark-gray solid as the stoichiometric compound iron(II) sulfide was not correct: but, only in the 1980s did researchers show that substance obtained was a mixture of phases (often called, rather loosely, 'non-stoichiometric' compounds). No attempt is made here either to summarize this research * or to explain its importance, simply because the science of phases is so frightfully complicated: however, do note that a line is placed over the nominal formula to indicate that a substance is non-stoichiometric, and that chemical equations are modified; e.g.,

The 'fall from grace' of iron(II) sulfide as the archetypal compound should, eventually, result in the removal of the suite mentioned above. Surprisingly, perhaps, there are very few alternatives which fulfil the two criteria of illumination and safety; of these, and by far the most attractive, is a suite involving the following substances: the elements zinc and iodine; a mixture of zinc and iodine; and the compounds water and zinc iodide. To summarize the results of this suite might well be regarded as profligate, but two cryptic 'cluettes' are surely in order.
The synthesis of aqueous zinc iodide is an exergonic process; i.e.,
![]()
By contrast, but with a delightful feeling of logic, the electrolytic decomposition of aqueous zinc iodide is an endergonic process; i.e.,
![]()
* See: J. K. Burdett and E. L. Miller, J. Am. Chem. Soc., 1987, 4081 (for a specific reference); F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, Wiley, New York, 1988 (for a concise summary).
Cela sert à faire bouillir la marmite
Collectively, living organisms use a variety of respiratory substrates as sources of chemical energy [including ethanol, glucose, and iron(II) sulfide]; of these, the most extensively used is glucose:
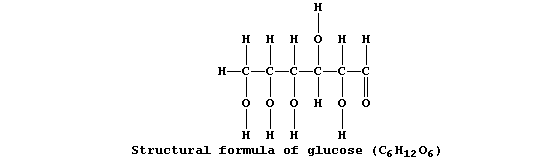
Equation 1, or its word equivalent, has been used in examination papers and textbooks to summarize one fermentation process involving glucose.
![]()
However, as written, equation 1 has little or no merit ...
First, and perhaps most importantly, equation 1 violates the Principle of the Conservation of Energy — which states 'energy is neither created nor destroyed, but can be transduced from one form to another'. All reactions involve an energy change (DE): but energy is never produced.
Second, equation 1 is often — but, admittedly, not always — stated to be a summary of the fermentation reaction: but this particular example of fermentation summarizes a biological process that involves at least eleven separate reactions.
Third, the inclusion of the word zymase is correct: but potentially misleading. Thus, by analogy with amylase and catalase, amongst others, one might reasonably assume that zymase is a single enzyme: whereas zymase is a biological mixture, extracted from yeast (Saccharomyces), that contains at least eleven enzymes.
And fourth, in the absence of state symbols, reactants are assumed to be in their normal states at room temperature. In the solid state, the rate of reaction between glucose and zymase would be immeasurably slow, because there would be remarkably few collisions between the particles.
The above (rather brutal) deconstruction would be of precious little value unless an acceptable alternative is available; happily, Equation 2 provides a most satisfactory summary of this fermentation process.
![]()
This fermentation process is but one example of respiration, whose purpose in a living organism is to release energy in the usable form of adenosine triphosphate (ATP). *
Accordingly, the use of Equation 3 to summarize this fermentation would be misleading, because it would imply that the purpose of respiration is to release heat energy.
![]()
Nevertheless, in most types of respiration, it is true that about 60% of the energy transduced (DE) is released as heat energy. #
* ATP, which is the immediate source of chemical energy for all living organisms, provides each living cell with the energy required for its endergonic processes (e.g., active transport and biosynthesis).
# During their (short-lived) flowering period, Arum lilies carry out a an alternative respiration process in which all the energy is released as heat; reproduction is clearly an 'energy sapping affair'.
AN INTRODUCTION TO ORGANIC CHEMISTRY
Preamble
A student often has one guardian or relative who considers it their duty in life to ensure that improving books occupy at least some space on the student's bookshelves ("... Thank you, Auntie. Just what I have always wanted. ..."). Should a Year 10 or 11 student wish to
possess the finest 'coffee-table' Chemistry book, then he or she would be well advised to direct their 'guardian angel' towards P. W. Atkins' Molecules, Scientific American Library, New York, 1987.
Diversity
Carbon has an unparalleled ability to form stable chains, rings, inter-connected rings, and a wide range of single and multiple bonds (e.g., C=C, C=N, C=O, C=S, CºC, and CºN). Accordingly, despite carbon being only the 17th most abundant element in the Earth's crust (0.09%), it is not surprising that organic compounds are ubiquitous and so numerous. [All compounds of carbon are referred to as organic compounds: except, by tradition, carbon dioxide, carbon monoxide, carbon disulfide, and various carbonates, cyanides, and hydrogencarbonates.] Certainly, the
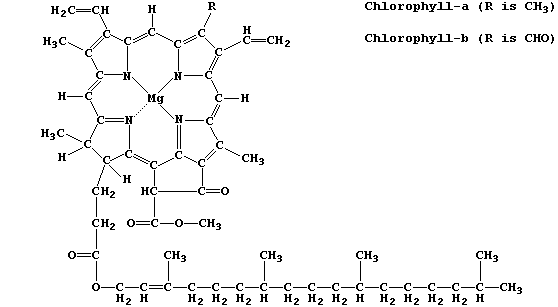
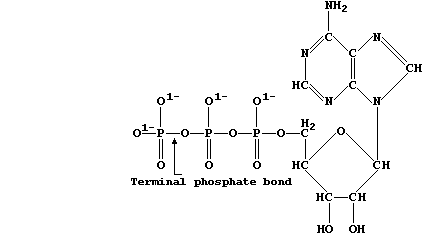
extraordinary diversity of living organisms is directly attributable to the facility of carbon atoms to form large and complex structures, as exemplified by chlorophyll-a and adenosine triphosphate (ATP) — two of the most important molecules in the biosphere.
Chlorophyll-a is the commonest photosynthetic pigment; its function is to absorb light energy and transduce it into chemical energy.
Adenosine triphosphate is the immediate source of chemical energy for all living organisms; the energy required for the endergonic processes in cells, such as active transport and biosynthesis, is released via enzymic hydrolysis of ATP's terminal phosphate bond.
Nomenclature
The systematic name of an organic compound is divided into two parts: one part indicates the chain length, ring size, or type of ring, and the other indicates the functional group(s). A functional group is an atom or group of atoms bonded to the carbon skeleton, and its reactions will often determine the chemical properties of the whole compound.
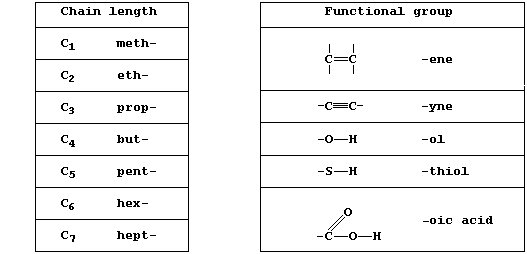
[Note, these two Tables present only a few of the prefixes and
suffixes used in the systematic naming of organic compounds.]
A group of compounds are said to belong to the same homologous series if they have the same functional group but different chain lengths or ring sizes. Within a series, homologs usually show similar chemical properties but different physical properties; e.g., all carboxylic acids are neutralized by alkalis,
![]()
but their boiling points steadily increase with increasing molar mass:
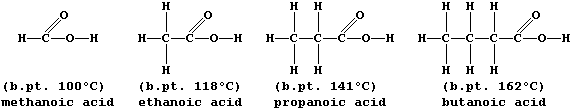
The molecular formulas of adjacent members of a series differ by CH2, so each homologous series can be represented by a general formula (this is CnH2nO2 for chain carboxylic acids). Finally, there are compounds with the same molecular formula but different structural formulas — these are known as isomers.
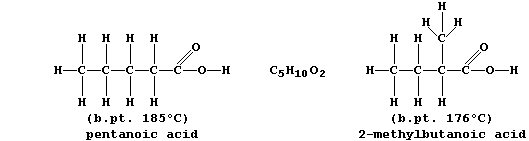
Petroleum
Petroleum, which was formed over millions of years by the compaction of dead organisms and the anaerobic decomposition of their biological molecules, is usually found trapped beneath impermeable cap rock and above a lower dome of sedimentary rock.
This oil, a non-renewable 'fossil fuel', is a mixture of solid, liquid, and gaseous hydrocarbons (mostly alkanes). In refineries, crude oil is fractionally distilled — a physical process which separates the various components on the basis of their different boiling points:
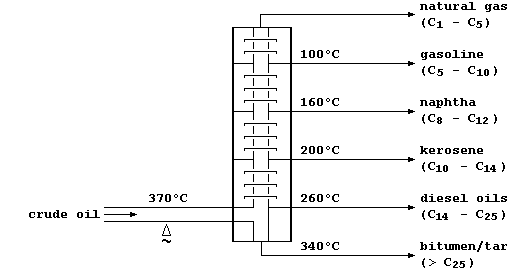
Some of these hydrocarbon fractions are used directly; e.g., as fuels (natural gas), or to manufacture petrol (gasoline), or to surface roads (tar). But, as this double bar-graph exemplifies, the demand for these fractions rarely matches their relative amounts in typical crude oil.
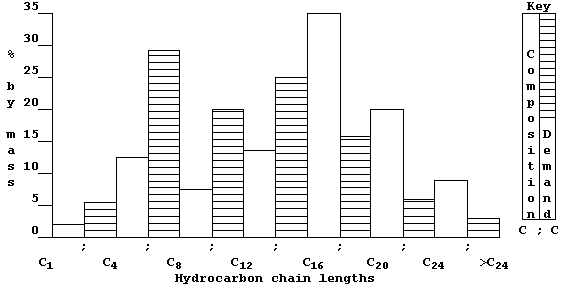
So, to overcome this economic problem, industry takes certain fractions and subjects them to catalytic cracking — a chemical process whereby large molecules are broken down by heat and by catalysis into smaller (and more useful) molecules; e.g.,
![]()
Mixtures of cracked products are separated by fractional distillation, and the fractions used as fuels, solvents, or synthetic intermediates.
Reaction Types
The biological and industrial importance of organic compounds, together with their structural diversity, has resulted in the development of hundreds of synthetic methods. Happily, most of these can be classified into just eight reaction types:
Addition (A), Condensation (C), Elimination (E), Hydrolysis (H),
Isomerization (I), Oxidation (O), Reduction (R), and Substitution (S).
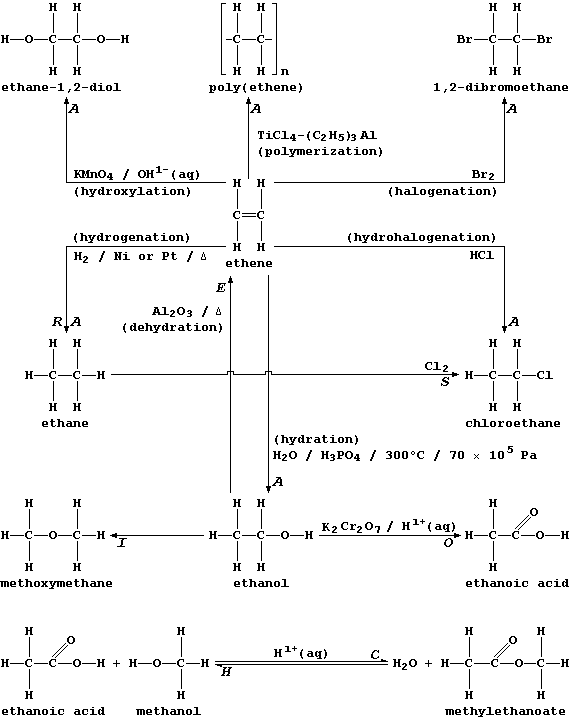
Two points are worth keeping in mind when considering the exemplars of each reaction type shown above. First, more than one description can be in common use for a given type; e.g., what is, formally, the addition of hydrogen to ethene will also be described as the reduction or the hydrogenation or the saturation of ethene. And second, each type can be usefully viewed as one of a 'pair', as exemplified explicitly above by addition-elimination and by condensation-hydrolysis.
Dr. R. Peters: Home Page; Aufbau1 Chemistry Resource;
Comedy-Dramas for Year 10-12 Students in British Orthography:
No. 1; No. 2; No. 3; No. 4; No. 5; No. 6; No. 7; No. 8; No. 9.