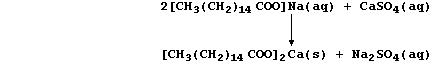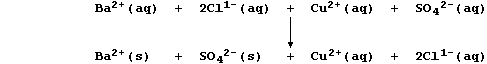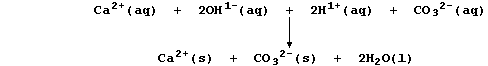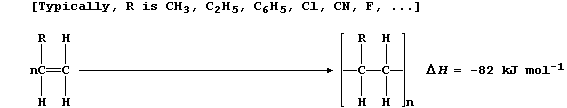AUFBAU1 [METALS]
[01] INTRODUCTION - REACTIVITY (1)
[02] INTRODUCTION - REACTIVITY (2)
[03] DISPLACEMENT REACTIONS (1)
[04] EXTRACTION of SODIUM
[05] CALCIUM & MAGNESIUM
[06] EXTRACTION of ALUMINIUM
[07] EXTRACTION of ZINC
[08] EXTRACTION of IRON
[09] COBALT
[10] TIN & LEAD
[11] EXTRACTION of COPPER
[12] DISPLACEMENT REACTIONS (2)
[13] DISPLACEMENT REACTIONS (3)
[14] PRECIPITATION REACTIONS
[15] LIGAND-EXCHANGE REACTIONS
[16] DISPLACEMENT REACTIONS (4)
[17] SODIUM COMPOUNDS
[18] ALUMINIUM & ZINC COMPOUNDS
[19] IRON COMPOUNDS
[20] COPPER COMPOUNDS (1)
[21] COPPER COMPOUNDS (2)
[22] MERCURY
[23] SILVER
[24] STRONTIUM
[25] CADMIUM
[26] MOLYBDENUM
[27] VANADIUM
[28] TITANIUM
[29] NICKEL
[30] MANGANESE
[31] CHROMIUM
[32] PLATINUM
METALS: INTRODUCTION - REACTIVITY (1)
An element's metallic character can be precisely defined in terms of
electrical conductivity, as exemplified by the sub-set shown below:
[Ag > Cu > Al > Ca > Mg > Na > Zn > K > Fe > Sn > Pb > Hg]
However, an element's metallic character is more usually considered in
terms of chemical reactivity; and, as exemplified by the same sub-set,
there is no apparent correlation between conductivity and reactivity:
[K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > Cu > Hg > Ag]
There is just the 'small' matter of defining chemical reactivity ...
Bon gré, mal gré?
The addition of a small quantity of solid potassium to water results in
the molten metal whizzing over the water's surface and the evolved gas
catching fire with a lilac flame. By contrast, a similar experiment with
solid lithium merely results in steady evolution of the gas (dihydrogen).
[Scene. A (kangaroo?) court in the state capital of Laputa]
Prosecutor: M'Lud. These results prove, beyond any reasonable doubt,
that Mr. Potassium is more reactive than the defendant.
Judge: Thank you kindly, Sir. [He addresses the prosecutor.] A
splendid case for the prosecution, if I may so say. Has
the defendant anything to add before I pass sentence?
Mr. Lithium: Yes, my Lord. I do admit that I have a higher activation
energy: but, my reaction with water is more exothermic
than Mr. Potassium's.
Judge: Stop! This Court will not be blinded by science ...
Mr. Lithium: But ...
Judge: Silence! You are in contempt of this Court. [He places
a black handkerchief over his grubby-looking, moth-eaten
wig.] Mr. Lithium, I sentence you, for the rest of your
natural life, to a position below Mr. Potassium in the
Reactivity Series. [Cheers from the public gallery.] |
The explanation for the observations above is as follows. Potassium has
a low melting point, 63°C, and so the heat of reaction is sufficient to
make it melt; the molten metal spreads out to expose a larger surface
area, and so it reacts even faster: as a result, heating in situ causes
the dihydrogen gas to catch fire. By contrast, lithium reacts much more
slowly, because it has a higher melting point, 181°C, and so there are
fewer collisions between the particles; its reaction with water is more
exothermic, but this heat energy is released more slowly. These energy
level diagrams provide an alternative summary of this explanation.
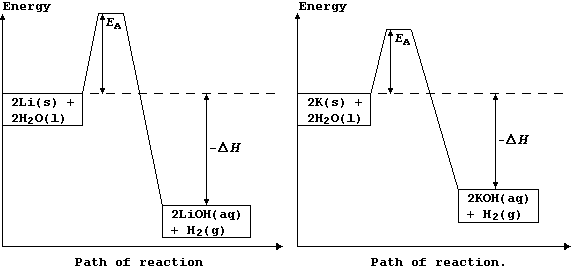
[Scene. The re-trial in the same (kangaroo?) court of Laputa]
Counsel: M'Lud. These energy level diagrams are important new
evidence; they prove, beyond any reasonable doubt, that
my client is more reactive than Mr. Potassium.
Judge: Thank you most kindly, Sir. [He addresses counsel.] I am
obliged to say that you have presented a waterproof case
for the defence. [The legal beavers humour the judge by
smiling weakly.] A clear miscarriage of justice. Has the
defendant anything to add before I overturn the original
verdict?
Mr. Lithium: Indeed I do, my Lord. In contrast to Mr. Potassium, I
react readily with dinitrogen. Nevertheless, with most
other reactants, I am less ...
Judge: Please do stop! Time, or at least my time, is valuable.
[He glances briefly, but wistfully, at his golf clubs.]
This Court will not be blinded by yet more science ...
Mr. Lithium: But ...
Judge: Silence! You are in contempt of this Court. [He places
a white handkerchief over his (now) grubbier-looking,
moth-eaten wig.] Mr. Lithium, I place you in a position
above Mr. Potassium in the Reactivity Series. [Cheers
from the (clearly undiscriminating) public gallery.] |
Indirectly, the reactions of lithium and potassium 'crystallize' two
problems which bedevil chemists, whether they be putative or mature.
First, the almost irresistible tendency to blur the distinction between
the rate and the energy change of a reaction. This blurring appears to
establish strong roots at an early stage in one's scientific career: so
much so, that authors of university and specialist textbooks invariably
feel the need to remind their readers of the distinction. *
And second, perhaps in the desire 'to hammer the subject into shape', a
tendency to assume that a limited number of reactions will necessarily
establish a rule which can be applied willy-nilly (bon gré, mal gré).
Neither of these problems are likely to disappear in the near future:
but their adverse effects can certainly be minimized by bearing in
mind four principles.
First, there is no connection between the activation energy and the
heat energy change (DH) for a chemical reaction.
Second, to ensure consistency, chemical reactivities should be compared
only in terms of energy (DE) or heat energy (DH) changes. #
Third, despite inevitable limitations in its use, a (metal) reactivity
series based on standard redox potentials is inherently self-consistent
because it is derived from measured physical data.
And fourth, a metal (M) reactivity series, based on redox potentials in
aqueous solutions, and a Periodic Table of the Elements, which is just
a method of summarizing the ground-state electronic structures of
gaseous atoms, both provide a suitable focus for correlation: but,
neither should be considered as a substitute for experimental facts.
* See, for example, F. A. Cotton and G. Wilkinson, Advanced Inorganic
Chemistry, Wiley, New York, 1988; P. W. Atkins, Physical Chemistry,
Oxford University Press, Oxford, 1989; and, P. J. Sykes, A Guidebook
to Mechanism in Organic Chemistry, Longman, London, 1986.
# Acceptance of this principle does not preclude comparing the rates
of reactions. [In advanced studies, reactivities are compared in terms
of kinetic and thermodynamic stabilities, which are discussed in terms
of activation energies and free energy changes (DG), respectively.]
METALS: INTRODUCTION - REACTIVITY (2)
A healthily critical attitude to reactivity can be acquired from the
careful consideration of the following example of hypothesis testing.
[Li > K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag]
Quelle horreur!
Hypothesis: 'Within each group of the Periodic Table, the reactivities
of the metallic elements (M) increase with increasing atomic number.'
Data: A. J. Bard et al., Standard Potentials in Aqueous Solutions,
Marcel Dekker, New York, 1985.
Results: Standard oxidation potentials derived from this source of data
show that the ease of oxidation for the metallic elements of six groups
is as follows.
Group 1: 3Li > 37Rb ³ 19K ³ 55Cs > 11Na
Group 2: 56Ba > 38Sr > 20Ca > 12Mg > 4Be
Group 3: 57La > 39Y > 21Sc
Group 11: 29Cu > 47Ag > 79Au
Group 12: 30Zn > 48Cd > 80Hg
Group 13: 13Al > 31Ga > 49In > 61Tl
Sources of Error: Surprisingly, reference books reveal a wide variation
in the numerical values of redox potentials. Nevertheless, in terms of
the ordering within groups, such books are concordant with Bard et al.
Conclusion: As adjudged by standard oxidation potentials, in a study
limited to the elements in Periods 2 to 6, the data provide no general
support for the hypothesis. Thus, Group 1 is irregular; the ease of
oxidation does increase as the atomic number increases in both Groups 2
and 3 (i.e., as each group is descended): but, the ease of oxidation
decreases as the atomic number increases in Groups 11, 12, and 13.
However, the use of different criteria for reactivity may well provide
support for this hypothesis. *
After due consideration of the above example of hypothesis testing, a
student may well exclaim: "Quelle horreur! Is there no method to this
madness?" The answer is yes: if, and only if, one accepts that many
patterns in Chemistry are complex, and that they start to emerge only
after focusing closely on the details.
Plus horreur?
A preliminary insight into some of the variables which determine the
reactivity of metals can be gained by placing a specific reaction type
'under the microscope'; e.g., reaction of a solid metal with an aqueous
solution of acid to form a hydrated metal cation and dihydrogen gas:
M(s) + H1+(aq) ———————————® M1+(g) + ½H2(g)
This reaction can be divided into several ergonic processes; the Table
below shows typical values of these processes for four metals known to
form uni-positive cations.
Ergonic process / kJ mol-1
|
Li |
K |
Na |
Ag |
M(s) ———————————® M(g) DH1 |
161 |
90 |
109 |
289 |
M(g) ———————————® M1+(g) + e- DH2 |
520 |
419 |
496 |
731 |
M1+(g) ——————————® M1+(aq) DHH |
-523 |
-331 |
-419 |
-464 |
H1+(aq) + e- ————® ½H2(g) DHR |
-432 |
-432 |
-432 |
-432 |
M(s) + H1+(aq) ——® M1+(aq) + ½H2(g) DH |
-274 |
-254 |
-246 |
+124 |
DH = DHS + DH1 + DH2 + DHR, where: DH1 is the heat of sublimation;
DH2 is the 1st ionization energy; DHH is the heat of hydration of
the metal cation; and, DHR is the heat of reduction of the aqueous
hydrogen ion to molecular dihydrogen. |
A complete explanation of these tabulated data is well beyond the scope
of this text: but even a partial explanation should be illuminating ...
Two processes are always endothermic, the heat of sublimation (DH1) and
the 1st ionization energy (DH2), whereas two are always exothermic, the
heat of hydration of the metal cation (DHH) and the heat of reduction
of the aqueous hydrogen ion to molecular dihydrogen (DHR). Because the
value of DHR is constant, the differences in the overall heat energy
change (DH) depend upon the relative magnitudes of DH1, DH2, and DHH.
The heat of sublimation reflects the strength of metallic bonding in the
solid state. However, the relationship between this bonding and ground-
state electronic structure of a gaseous atom is very complex indeed. Be
that as it may, uniform periodicity is clearly not observed; thus,
3Li > 11Na > 19K (Group1), but 79Au > 29Cu > 47Ag (Group 11).
The 1st ionization energy is the only ergonic process which is directly
related the ground-state electronic structure of a gaseous atom.
Nevertheless, here also, uniform periodicity is not observed; thus,
3Li > 11Na > 19K (Group1), but 79Au > 29Cu > 47Ag (Group 11).
The heat of hydration reflects the electronic structure of the gaseous
cation and its subsequent bonding with a (variable) number of water
molecules to form the hydrated cation. Unfortunately, a clear statement
of periodicity is precluded because, in contrast to those in Group 1,
the metals in Group 11 show variable oxidation states.
And finally, ... These data would support this principle: 'high metal
reactivity is favoured by a low heat of sublimation, a low ionization
energy, and a high heat of hydration of the metal cation'. The overall
heat energy change (DH) is negative for each Group 1 metal, and the
relative magnitudes of DH parallel the measured energy changes of their
reactions with water (i.e., Li > K > Na); the 'anomalous' position of
lithium is attributable to the higher heat of hydration of the lithium
cation. In contrast, largely because of silver's much higher ionization
energy, DH is positive for this Group 11 metal; this is consistent with
the observed lack of reaction between silver and dilute acid or water.
[Scene. A court in the state capital of Poppermania]
Mermaid: Plus horreur! ... M'Lud ... [The judge holds up his hand.]
Judge: My Lord is the correct method of address, if you please.
Mermaid: Sorry, my Lord. These results and explanations are all well
and good: but, they can have little relevance to solid ionic
compounds, such as chlorides, oxides, and nitrides, surely?
Judge: [He smiles tolerantly at this less-than-humble student.]
I do admit, strictly speaking, only parts are; for example,
the DH1 and DH2 terms. However, for other reactants, there
should be analogous terms to DHH and DHR. Perhaps you would
care to note the relationship of DHH to the lattice energy?
Mermaid: Duly noted, M'Lud ... I mean, my Lord. Please no more ...
My time is valuable! [She states petulantly before glancing
briefly, but proudly, at her varnished finger-nails.]
Judge: Oh dear, in my day ... no, never mind. [He sighs.] Remember,
Rome was not built in a day: although it may well have burnt
down in one! [He glances at his fiddle before dabbing some
eau-de-cologne on his pristine wig.] Mermaid, I ... advise
you to spend at least part of your free-time giving the grey
cells some aerobic exercise. [Sighs of relief from the rest
of the synchronized-swimming team in the public gallery.]
Mermaid: My Lord, perhaps I should emigrate to Laputa? [... but the
venerable judge has fallen asleep (bless his silken socks).] |
* The student is encouraged, albeit gently, to consider testing this
hypothesis, or a similar one which focuses on Periods, using different
physical data (e.g., heats of sublimation, ionization energies, energy
changes for the formation of solid ionic compounds, ...); such data
should be abstracted from reference books.
METALS: DISPLACEMENT REACTIONS (1)
From a consideration of a reactivity series based on redox potentials,
one might reasonably predict that a metal should displace hydrogen from
either water or dilute acids if it is more reactive than hydrogen ...
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. The Table summarizes the results of experiments which examined the
reactions of eleven metals with water and/or steam, whereas the diagram
shows the apparatus used for the experiments with steam.
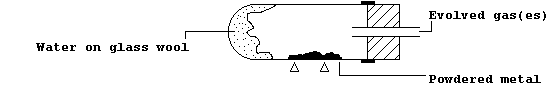
|
Observations of reactions of metals with water and/or steam * |
K |
Violent reaction with cold water; metal floated on water as
a small molten ball; evolved gas burnt spontaneously with a
lilac flame; resulting solution turned universal indicator
paper blue-purple. |
Ca |
Very vigorous reaction with cold water; evolved gas produced
an explosive pop with a lighted splint; resulting milky-white
suspension, which was very hot, turned universal indicator
paper dark-blue. |
Na |
Very vigorous reaction with cold water; metal floated on water
as a small molten ball; evolved gas did not burn spontaneously,
but produced an explosive pop with a lighted splint; resulting
solution turned universal indicator paper blue-purple. |
Mg |
Little or no reaction with cold water. Very slow reaction with
hot water (containing traces of universal indicator solution);
the solution changed slowly from green to blue over a period
of two hours (but no precipitate formed). Rapid reaction with
steam; white solid formed; evolved gas burnt. |
Al |
No reaction with boiling water: although reaction with boiling
water containing a catalytic quantity of sodium chloride;
evolved gas burnt with an explosive pop. Fairly rapid reaction
with steam; white solid formed; evolved gas burnt. |
Zn |
No reaction with boiling water. Fairly slow reaction with
steam; initially formed yellow solid cooled to a white solid;
evolved gas burnt intermittently. |
Fe |
Very slow reaction with steam; black solid formed; gas, if
evolved, not in sufficient quantity to be burnt. |
Sn |
Little or no reaction with steam; trace of white solid formed. |
Pb |
Little or no reaction with steam; trace of white solid formed. |
Cu |
No (evidence for) reaction with steam. |
Ag |
No (evidence for) reaction with steam. |
* Caveat This Table presents descriptors which indicate both relative
reaction speeds & energy changes; this conflation must not be allowed
to obscure the fact that there is no connection between the activation
energy & the heat energy change (DH) for a chemical reaction. |
(a) In common with similar presentations in textbooks, these results
do not refer to controlled experiments. Thus, although the qualitative
independent variable chosen was 'metal', no attempts were made to keep
other variables constant; e.g., the volume of water or the absence of
catalysts. Name the two physical quantities of the metals which should
have been held constant. ______________________________________________
[2]
(b) The symbol equation for the reaction between potassium and water is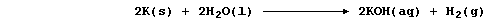
Noting that calcium and sodium react with water similarly, construct
the symbol equation for the reaction between calcium and water. _______
_______________________________________________________________________
[2]
Calcium hydroxide is an alkali, because it is a base which is soluble
in water (albeit only slightly soluble). What is the common name for
aqueous calcium hydroxide? ____________________________________________
[1]
(c) Magnesium reacts with steam to form the hydroxide,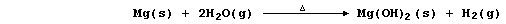
but this thermally decomposes at the temperature of the experiment,
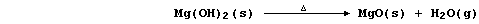
so the reaction between magnesium and steam is usually summarized as:
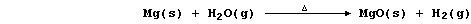
Noting that aluminium and zinc react with steam similarly, construct
the (overall) symbol equation for the reaction between zinc and steam.
_______________________________________________________________________
[1]
(d) Iron has been shown to react reversibly with steam; i.e.,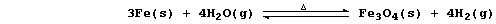
Note, however, that these particular experiments could not determine
the reversible character of these reactions, because the apparatus used
was an 'open system'. Name one substance which is transferred to the
surroundings in these experiments. ____________________________________
[1]
[Neither tin nor lead react with water or steam: but both react with
dilute acids. The explanation for these observations is, regrettably,
beyond the scope of this text; nevertheless, one should be aware that
redox potentials are dependent on pH.]
2. Readily duplicated investigations have shown that, under comparable
conditions, metals react faster with dilute acids than with water; a
partial explanation for this difference is as follows. Water undergoes
very slight dissociation (or ionization); i.e.,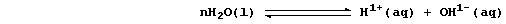
Because the position of the above equilibrium lies almost completely to
the left, the concentration of hydrogen ions in water is low [roughly
10-7 mol dm-³ (i.e., pH 7)]. By contrast, dilute acids undergo either
partial or complete dissociation; e.g.,
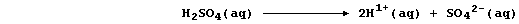
Therefore, the concentration of hydrogen ions in dilute acids is higher
[ranging from 10-1 to 10-6 mol dm-³ (pH 1 to 6)]. In consequence, there
will be more collisions between metal atoms and these ions in dilute
acids than in water, and so faster reactions.
Construct the symbol equation for the reaction between:
Sodium and dilute hydrochloric acid (dangerously explosive) ___________
_______________________________________________________________________
Magnesium and dilute sulfuric acid (vigorous) _________________________
_______________________________________________________________________
Aluminium and dilute sulfuric acid (fairly vigorous) __________________
_______________________________________________________________________
Iron and dilute sulfuric acid (which, in part, is analogous to that
between steel and this component of 'acid rain') ______________________
_______________________________________________________________________
[8]
3. Neither copper nor silver react with water, steam, or dilute acids.
Name another metal which is similarly unreactive. _____________________
[1]
METALS: EXTRACTION of SODIUM
Sodium, the fourth most abundant metal in the Earth's crust (2.4%), is
usually found as the chloride (e.g., in the ore rock salt or dissolved
in sea water). Sodium has a low melting point (98°C) and a low density
(0.97 g cm-³), which are characteristics of Group 1 metals, and forms
(usually) white or colourless compounds in only one oxidation state
(I), which is typical of main group metals.
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. Sodium is extracted by the electrolytic reduction of purified,
molten rock salt; the ionic equations for the reactions occurring at
the electrodes are:
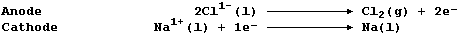
(a) Label this diagram of a Downs electrolytic cell with: Carbon anode;
Chlorine gas; Insulator; Molten electrolyte; Molten sodium; and, Steel
cathode.
(b) To lower the melting point of sodium chloride from 774°C to about
600°C, and so reduce the energy costs, calcium chloride is added to the
rock salt. As a result of this added impurity, a mixture of molten
sodium and solid calcium is obtained. Write an ionic equation for the
reduction of calcium ions at the cathode.
_______________________________________________________________________
[1]
(c) Water must not be present in the Downs cell, partly because a
different cathodic reaction occurs. Thus, hydrogen gas is evolved at
the cathode from the electrolysis of an aqueous solution containing
Na1+(aq), Cl1-(aq), H1+(aq), and OH1-(aq) ions. Write an ionic equation
for the reduction of hydrogen ions at the cathode.
_______________________________________________________________________
[1]
(d) After manufacture, sodium is stored under oil because it is rapidly
oxidized by atmospheric oxygen. Furthermore, sodium reacts explosively
with many other non-metallic elements and compounds. Construct the
symbol equation for the reaction of sodium with:
Difluorine ____________________________________________________________
Dioxygen ______________________________________________________________
Water _________________________________________________________________
[6]
Sodium's high reactivity has necessarily restricted its use to a few
applications. Thus, aside from its incorporation into specialized
alloys (e.g., Na-Hg amalgam), it is used in street vapour lamps, as a
coolant in nuclear reactors, and as a chemical reducing agent.
2. Sodium's use as a coolant in nuclear reactors depends on three of
the metal's properties. First, it is a liquid at temperatures between
98°C and 883°C, so the metal flows easily around the hot reactor core
(which operates at a temperature of about 660°C). Second, it is a good
thermal conductor (because of its free moving delocalized electrons),
so excess heat generated in the core is conducted away efficiently.
And third, it has a relatively high thermal capacity, so large amounts
of heat energy are absorbed for any given temperature rise.
[Q = n × z × F and Q = I × t, where: Q, measured in coulombs (C), is
the quantity of electricity; n is the number of moles of substance
evolved at the electrode; z is the charge on the ion; F is a constant,
with a value of 96500 C mol-¹; I, measured in amps (A), is the current;
and t, measured in seconds (s), is the time.]
(a) A fast-breeder reactor under construction required 690 tonnes of
sodium. A typical Downs electrolytic cell operates continuously at a
current of 25 kA. Determine the time (t) it would take to obtain the
required mass of sodium - as follows.
Convert this mass (m) of sodium from tonnes to grammes (where
1 tonne = 1000 kg). ___________________________________________________
Calculate the number of moles (n) in this mass (m) of sodium. _________
_______________________________________________________________________
Calculate the quantity of electricity (Q) required to deposit this
number of moles (n) of sodium at the cathode. _________________________
_______________________________________________________________________
And finally, calculate the time (t) taken for this quantity of
electricity (Q) to be used. ___________________________________________
_______________________________________________________________________
[7]
(b) The volume (V1) of one mole of any gas at room temperature (25°C =
298 K; T1) and pressure (0.1 MPa; P1) is 24 dm³; furthermore, the
following relationship holds true for gases:
P1 × V1 P2 × V2
¾¾¾¾¾ = ¾¾¾¾¾
T1 T2
Determine the volume (V2) of chlorine gas obtained, at room temperature
and high pressure (20 MPa; P2), during the production of this mass of
mass of sodium - as follows.
State the number of moles (n) of chlorine atoms, Cl(g), formed at the
anode. ________________________________________________________________
State the number of moles of chlorine gas, Cl2(g), evolved at the
anode. ________________________________________________________________
Calculate the volume of gas (V1) obtained at room temperature (T1) and
pressure (P1). ________________________________________________________
Then, using the above relationship, calculate the volume (V2) of gas
at the increased pressure (P2). _______________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
3. Sodium metal reduces liquid ammonia to sodium amide, NaNH2(s), a
sparingly used fertilizer. Construct the symbol equation for this redox
reaction, which is executed under anhydrous and anaerobic conditions.
_______________________________________________________________________
[2]
METALS: CALCIUM & MAGNESIUM
Calcium, (m.pt. = 839°C; r = 1.54 g cm-³), is the third and magnesium,
(m.pt. = 649°C; r = 1.74 g cm-³), is the fifth most abundant metal in
the Earth's crust (4.2% and 2.3%, respectively); as the Table below
shows, they occur in a variety of minerals and ores. In common with
several other Group 1 and 2 elements, both metals are often extracted
by the electrolytic reduction of their molten chlorides.
Name of mineral or ore |
Composition or principal component |
Gypsum |
CaSO4.2H2O |
Limestone, calcite, chalk, marble |
CaCO3 |
Dolomite |
CaCO3.MgCO3 |
Magnesite |
MgCO3 |
Epsomite |
MgSO4.7H2O |
Despite their relative abundances, neither metal is used extensively in
industry, although both are used in alloys and for chemical reductions:
nevertheless, in the long term, magnesium may replace aluminium as the
low-density structural metal of choice because the supply available in
seawater is virtually unlimited. By way of contrast, both calcium and
magnesium ions have been shown to be essential to all living organisms,
and both affect the 'hardness' of water (one aspect of water quality
considered to be important in developed countries).
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. The presence of nitrate ions (leached from farms), or toxic heavy
metal ions (leached from mines), or calcium ions (which contribute to
hardness), is rarely an important consideration of water quality in
developing countries - where survival frequently depends on obtaining
enough water which is sufficiently pathogen-free. When favourable
conditions prevail, water is purified by boiling (a process which kills
pathogens by irreversibly denaturing their enzymes). Name one pathogen
which is often present in water contaminated with untreated sewage.
_______________________________________________________________________
[1]
2. This diagram shows a simplified plan of a typical water-treatment
works in a developed country.
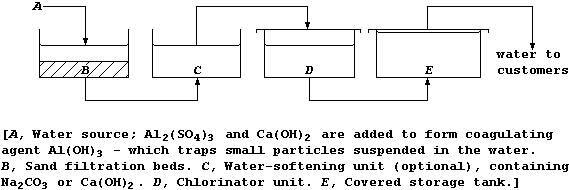
(a) Name one urban water source. ______________________________________
[1]
(b) Suggest the purpose of the:
Sand filtration beds __________________________________________________
Chlorinator unit ______________________________________________________
[2]
(c) Each storage tank must be covered. Suggest one biological reason
why sunlight should be prevented from reaching the water. _____________
_______________________________________________________________________
[1]
3. 'Hard water', which is water that does not readily form a lather
with soap, is caused by the presence of any dissolved Group 2 ions -
though the focus here is on those of calcium [i.e., aqueous Ca(II)] ...
Unpolluted rainwater is effectively a dilute solution of carbonic acid,
H2CO3(aq), and 'temporary' hard water results from the slow reaction of
rainwater with minerals or ores containing calcium carbonate: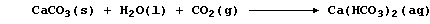
Water containing soluble calcium hydrogencarbonate is considered to be
temporarily hard because the hydrogencarbonate is thermally unstable;
thus, when it is thermally decomposed, soluble calcium ions are removed
from solution as a precipitate - commonly known as 'scale' or 'fur':
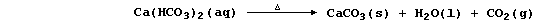
'Permanent' hard water results from the slow (and slight) dissolution
of the calcium sulfate present in minerals and ores:
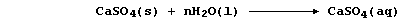
Water containing dissolved calcium sulfate is considered to be
permanently hard because this type of hardness cannot be removed by
boiling; indeed, removal of the soluble calcium ions requires the use
of an 'ion exchanger' or a 'water-softener' (e.g., sodium carbonate):
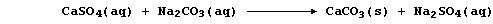
(a) Construct a single symbol equation, involving naturally occurring
magnesite, for the formation and removal of temporary hard water. _____
_______________________________________________________________________
[2]
(b) The structural formula of a typical soap, sodium hexadecanoate, is: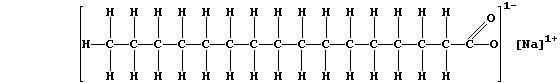
The anions of this soap, better known as sodium palmitate, react with
the calcium ions present in permanent hard water to form a precipitate
of 'scum':
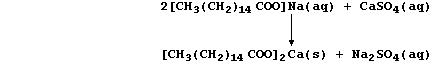
Complete a similar equation for the reaction of these anions with the
calcium ions present in temporary hard water:
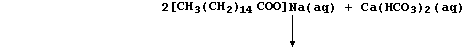
_______________________________________________________________________
[2]
(c) In hard water areas, scale precipitated in pipes and in tanks can
be removed safely by neutralization with an aqueous solution of a weak
acid [e.g., HCOOH(aq)]. Suggest and explain, using a symbol equation,
why farmers usually prefer to remove scale with dilute nitric acid. ___
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
4. State two biologically important rôles of calcium ions, apart from
the formation of exo- and endo-skeletons. _____________________________
_______________________________________________________________________
[2]
5. Name and state the function of one biological molecule that
contains magnesium ions. ______________________________________________
_______________________________________________________________________
[2]
METALS: EXTRACTION of ALUMINIUM
Aluminium, the commonest metal in the Earth's crust (8.3%), is usually
found as the oxide (e.g., in the ore bauxite). Typical of main group
metals, aluminium has a fairly low melting point (660°C), a low density
(2.7 g cm-³), and forms (usually) white or colourless compounds in only
one oxidation state (III).
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. Aluminium is extracted by the electrolytic reduction of purified
molten bauxite; the ionic equations for the reactions occurring at the
electrodes are:
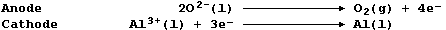
(a) Label this diagram of an industrial electrolytic cell with:
Graphite anode; Graphite cathode; Insulator; Molten aluminium; Molten
electrolyte; and, Solid crust of electrolyte.
(b) To lower the melting point of aluminium oxide from about 1500°C to
1000°C, and so reduce energy costs, cryolite (Na3AlF6) is added to the
bauxite. Explain why (pure) aluminium oxide has a high melting point.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
(c) Name one toxic gas that might arise from the thermal decomposition
of cryolite. __________________________________________________________
[1]
(d) The anode needs to be replaced periodically because the carbon is
completely oxidized by the evolving dioxygen. Name the toxic pollutant
formed by partial oxidation of the carbon anode. ______________________
[1]
[Q = n × z × F and Q = I × t, where: Q, measured in coulombs (C), is
the quantity of electricity; n is the number of moles of substance
evolved at the electrode; z is the charge on the ion; F is a constant,
with a value of 96500 C mol-¹; I, measured in amps (A), is the current;
and t, measured in seconds (s), is the time.]
2. In industry, a typical electrolytic cell operates continuously at a
current of 4000 kA. Determine the mass (m) of aluminium that forms at
the cathode every day - as follows.
Calculate the quantity of electricity (Q) used every 24 hours. ________
_______________________________________________________________________
Calculate the number of moles (n) of aluminium formed at the cathode.
_______________________________________________________________________
And finally, calculate the mass (m) of aluminium formed at the cathode.
_______________________________________________________________________
[6]
3. Electrolytic reduction, which is often used for the extraction of
the most reactive metals, requires expensive electrical energy: by
contrast, chemical reduction with carbon uses a relatively cheap source
of chemical energy. Which method of generating electrical power is the
cheapest and most environmentally acceptable? _________________________
[1]
297 kJ of energy are required to produce 1 mole of aluminium metal by
electrolysis, whereas only 26 kJ of energy are required to recycle
1 mole. Calculate the percentage energy saved by recycling aluminium.
_______________________________________________________________________
[2]
4. Aluminium's high natural abundance, high resistance to corrosion,
high thermal conductivity, low density, and low electrical resistance,
has resulted in its widespread use (e.g., in window frames, cooking
utensils, food and drink cans, aircraft, and electrical power lines).
The metal surface is invariably covered by a very thin layer of oxide,
because pure aluminium is rapidly oxidized by atmospheric oxygen;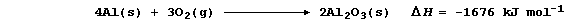
This layer is non-porous and protects the metal from further corrosion,
providing it is not exposed to aqueous chloride ions.
(a) Paralleling aluminium's increased use in cooking utensils and in
food containers, there has been an increase in the number of people who
suffer from Alzheimer's disease. This parallel may indeed be a most
unfortunate coincidence, rather than a correlation, but one partial
explanation could be as follows. Food is often preserved in brine and
cooked with salt; so, should the protective layer of aluminium oxide in
the containers be removed by aqueous chloride ions, soluble aluminium
ions could be formed, ingested, and then absorbed into the bloodstream.
Construct an explanation, complete with two symbol equations, for the
assertion that it could be hazardous to use aluminium foil when cooking
salted meat in the presence of steam. _________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
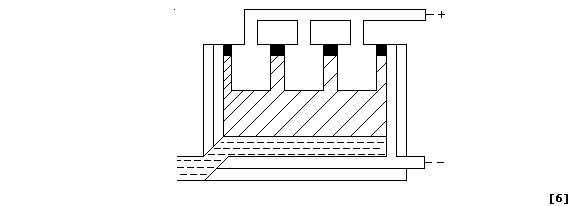
(b) When the thin layer of oxide is made thicker, by anodizing, then
it can easily absorb variously coloured dyes. Shown below is a diagram
of a typical electrolytic cell used to anodize aluminium objects.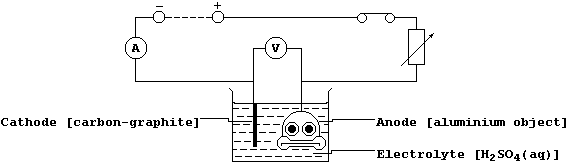
Oxygen gas is evolved at the anode from the electrolysis of an aqueous
solution containing H1+(aq), OH1-(aq), and SO42-(aq) ions; the ionic
equations for the reactions occurring at the electrodes are:

The aluminium object immediately combines with this evolved oxygen gas
to form more aluminium oxide on its surface. Suggest two reasons why
aluminium is anodized. ________________________________________________
_______________________________________________________________________
[2]
METALS: EXTRACTION of ZINC
Zinc, which is relatively rare in the Earth's crust (0.007%), occurs
mainly as the sulfide or the carbonate (e.g., in the ores zinc blende
and calamine, respectively). This element is not considered to be a
transition metal, despite its position on the Periodic Table, partly
because it forms compounds which are (usually) white or colourless and
in only one oxidation state (II).
[.. K > Ca > Na > Mg > Al > Zn > Cd > Fe > Sn > Pb > (H) > Hg > Cu ..]
1. Extracting natural resources invariably produces a conflict between
economic advantages and conservation of the environment. For example,
open-cast mining of zinc ores usually provides employment for the local
population: but also produces large quantities of waste rock (known as
spoil), as well as the disruption or destruction of delicately balanced
ecosystems. Suggest one conservation measure which should be undertaken
when resources are extracted. _________________________________________
_______________________________________________________________________
[1]
2. Zinc is extracted and purified by the processes summarized in this
flow diagram.

(a) Combustion of crude zinc blende usually results in a mixture of
metal and silicon oxides, because the sand-bearing ores of zinc, lead,
and cadmium often occur together. Construct the symbol equation for the
complete combustion of (pure) zinc sulfide. ___________________________
_______________________________________________________________________
[2]
State the most important use of the by-product (i.e., sulfur dioxide).
_______________________________________________________________________
[1]
(b) Construct the symbol equation for the neutralization reaction of
zinc carbonate and dilute sulfuric acid. _____________________________
_______________________________________________________________________
[2]
(c) In the electrolytic reduction of aqueous zinc sulfate, which
contains the ions Zn2+(aq), SO42-(aq), H1+(aq), and OH1-(aq), dioxygen
is evolved at the carbon-graphite anode and zinc is deposited at the
carbon-graphite cathode. Write an ionic equation for the reaction which
occurs at the:
Anode _________________________________________________________________
Cathode _______________________________________________________________
[3]
Suggest and explain one reason why zinc is not extracted by the
electrolytic reduction of zinc oxide. _________________________________
_______________________________________________________________________
[2]
(d) Impure zinc is obtained when a mixture of oxides, obtained from the
combustion of crude zinc blende, is heated with coke and limestone in a
furnace at a temperature of about 1000°C. Fairly pure zinc, separated
from impurities by physical methods (e.g., fractional distillation), is
then either used directly or further purified electrolytically.
Coke, the source of chemical energy in the blast furnace, is burnt both
to release heat energy and to provide the main reducing agent:
Calcium oxide, formed by thermal decomposition of limestone, reacts
with silicon oxide to form slag (which is less dense than molten lead):
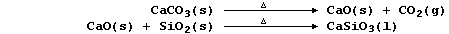
|
Zinc |
Cadmium |
Lead |
Melting point / °C |
420 |
321 |
328 |
Boiling point / °C |
908 |
765 |
1751 |
Density (r) / g cm-³ |
7.14 |
8.64 |
11.34 |
Noting the properties given in the Table, label this diagram of a zinc
blast furnace with: CaSiO3(l); Cd(l); Condenser; Hot air blast; Pb(l);
Reactants (oxides, coke, and limestone); Waste gases; and, Zn(l).
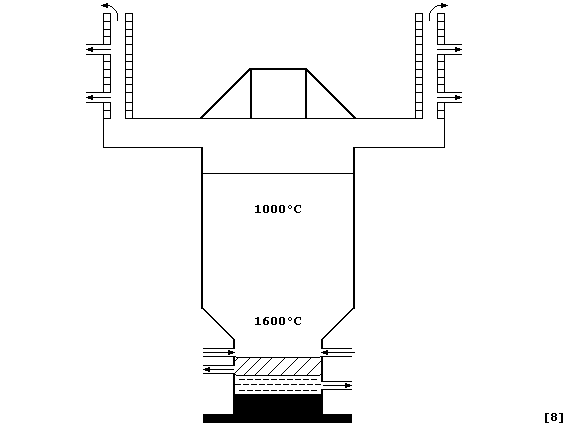
Construct an explanation, complete with two symbol equations, why
calamine could be used directly as a raw material in the blast furnace.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
What two physical processes are involved in fractional distillation?
_______________________________________________________________________
[2]
3. Zinc is used in alloys, in batteries, and to galvanize iron. If the
trends in present-day use of zinc continues, it has been guesstimated
that the known reserves of zinc ores will last for perhaps no more than
20 years. Suggest two ways of overcoming this potential shortage of
zinc. _________________________________________________________________
_______________________________________________________________________
[2]
METALS: IRON
Iron, the second most abundant metal in the Earth's crust (5.6%), is
usually found as an oxide (e.g., Fe2O3 and Fe3O4 in the ores haematite
and magnetite, respectively). This element, which has been shown to be
essential to all biological species, is a typical transition metal.
Thus, iron has a high melting point (1535°C) and a high density
(7.87 g cm-³), forms coloured compounds (which are often pale-green or
brown), and shows variable oxidation states [e.g., Fe(II) and Fe(III)].
Furthermore, iron and many of its compounds show catalytic activity
(e.g., iron powder is used in the Haber synthesis of ammonia, and
several iron-containing enzymes are involved in nitrogen-fixation, in
photosynthesis, and in respiration).
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. Iron is extracted from its ores by the chemical reduction of iron
oxides with carbon in a furnace at a temperature of about 800°C;
overall, the processes can be summarized by these equations:
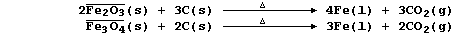
Coke, the source of chemical energy in the blast furnace, is burnt both
to release heat energy and to provide the main reducing agent:

Calcium oxide, formed by thermal decomposition of limestone, reacts
with the silicon oxide present in sand, a major impurity in iron ores,
to form slag (which is less dense than molten iron):
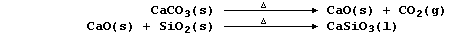
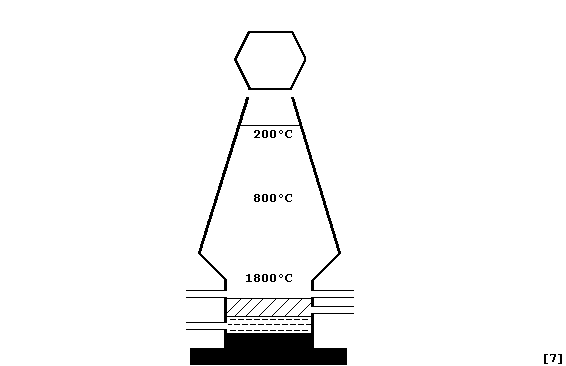
(a) Label this diagram of an iron blast furnace with: Blast of hot air;
Molten iron; Molten slag; Outlet for iron; Outlet for slag; Reactants
(iron ore, coke, and limestone); and, Waste gas outlet.
(b) Various iron oxides are formed within the furnace. Construct the
symbol equation for the reduction of iron(II) oxide by carbon monoxide.
_______________________________________________________________________
[2]
(c) Name two of the waste gases. ______________________________________
[2]
2. Nearly all metals corrode: but only iron 'rusts'. The rusting of
iron, which starts with the anodic reaction Fe(s) ——® Fe2+(aq) + 2e-,
involves a complicated series of redox and precipitation reactions that
can be loosely summarized by the following equation (where, n and x are
variable numbers, and z and y are constants):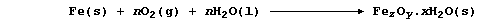
(a) Laboratory analysis showed that a sample of rust contained 5.6 g of
iron, 2.4 g of oxygen, and 2.7 g of water. The steps involved in this
analysis included strong heating of the hydrated oxide, to remove its
water of crystallization, followed by strong heating of the anhydrous
oxide in a stream of dihydrogen, to effect its reduction; i.e.,
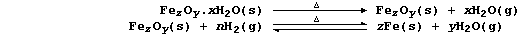
Time (t) / s |
0 |
60 |
120 |
180 |
180 |
180 |
240 |
300 |
Cu deposited / mg |
0 |
110 |
210 |
250 |
315 |
325 |
430 |
530 |
Cu deposited (n) / mmol |
0.0 |
1.7 |
3.3 |
3.9 |
4.9 |
5.1 |
6.7 |
8.3 |
Complete the Table above, so as to determine the empirical formula of
this sample of rust. __________________________________________________
[5]
Rusting occurs faster in the presence of aqueous chloride ions; a
partial explanation for this observation is as follows. Corrosion is an
electrochemical process, in which one part of the metal surface acts as
the anode, another part acts as the cathode, and an aqueous electrolyte
completes the electrical cell. Electron flow from anode to cathode is
facilitated by migration of ions such as Fe2+(aq) in the electrolyte;
and, because pure water is only very slightly ionized, an aqueous
solution of chloride ions is clearly a better electrolyte than water.
(b) State two ions present in 'acid rain' that might also increase the
rate of rusting / corrosion. __________________________________________
[2]
Iron's widespread use as a structural material is due to its low cost
and high tensile strength. Unfortunately, rust weakens structures;
moreover, it cannot protect the metal from further corrosion because it
is porous. Accordingly, apart from important safety considerations, the
costs of possible replacements need to be minimized. And so, when iron
(or steel) is likely to rust, it is: painted, coated with plastic, or
plated with another metal, all of which provide a physical barrier; or
attached to a more reactive metal, which acts as a 'sacrificial anode'.
(c) Zinc is more easily oxidized than iron. Therefore, in an electrical
cell, the preferred anodic reaction Zn(s) ——® Zn2+(aq) + 2e- results in
electron flow to an iron cathode, and so prevents the anodic reaction
Fe(s) ——® Fe2+(aq) + 2e-. Thus zinc acts as a sacrificial anode, and so
blocks of zinc attached to iron structures provide effective protection
against rusting. Explain, in similar terms, the effectiveness of:
Magnesium blocks ______________________________________________________
_______________________________________________________________________
_______________________________________________________________________
Tin blocks ____________________________________________________________
_______________________________________________________________________
[5]
(d) Iron coated with a layer of zinc is known as 'galvanized iron'.
Galvanization protects iron from rusting, as follows: the layer acts as
a physical barrier to dioxygen and water; and then the zinc acts as a
sacrificial anode when this layer is breached. Suggest one disadvantage
in galvanizing iron. __________________________________________________
[1]
METALS: COBALT
Cobalt, usually found in the Earth's crust as a sulfide (e.g., in the
ore cobaltite), is the second rarest (0.003%) of the four elements that
can be magnetized; the other three are iron (5.6%), nickel (0.008%),
and gadolinium (0.0005%). This relative rarity means that cobalt is not
used for structural purposes but for the formation of specialized
alloys (e.g., Alnico, which is used to manufacture permanent magnets).
This transition metal, which has a high melting point (1495°C) and a
high density (8.89 g cm-³), forms compounds in several oxidation states
[e.g., (blue) cobalt(II) chloride and (brown) cobalt(III) chloride].
[.. K > Ca > Na > Mg > Al > Zn > Fe > Co > Sn > (H) > Cu > Hg > Ag ..]
1. The extraction of cobalt from its ores is complex, but the final
process usually involves chemical reduction of cobalt(II) dicobalt(III)
oxide (Co3O4). Construct the symbol equation for the reduction of this
tetroxide with each of these reducing agents used in industry:
Aluminium _____________________________________________________________
Carbon ________________________________________________________________
Dihydrogen ____________________________________________________________
[6]
Cobalt can be electrolytically purified, using impure cobalt as the
anode, pure cobalt as the cathode, and aqueous cobalt(II) chloride as
the electrolyte. Explain why solid cobalt(II) chloride would not be
suitable as an electrolyte. ___________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
2. Vitamin-B12, which is synthesized exclusively by bacteria (e.g.,
Methanobacteria), has a structure similar to both chlorophyll-a and
haemoglobin.
This compound is involved in the bacterial biosynthesis of methane and
organometallic compounds of heavy metals (e.g., dimethylmercury).
Vitamin-B12 is also a vital cofactor in the synthesis of mammalian red
blood cells; it is acquired from dietary sources and from symbiotic
bacteria residing in the alimentary canal. Name the human deficiency
disease caused by a lack of this vitamin. _____________________________
[1]
3. Cobalt(II) chloride is used as an indicator of water; thus, in the
presence of this compound, blue 'cobalt(II) chloride paper' turns pink: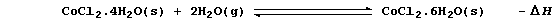
State and explain, using Le Chatelier's Principle, the effect of an
increase in temperature on this reaction. _____________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
4. Anhydrous cobalt(II) chloride is an effective catalyst for the
synthesis of alkanes (in non-aqueous solvents); a typical scheme is: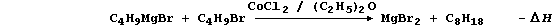
(a) Construct a similar scheme for the synthesis of pentane (C5H12).
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) Complete and label the energy level diagram for this synthesis of
pentane.
Energy ___
___
___________________ _ _ _ _ _ _ _ _ _ _ _ _
___________________
_______________
_______________
___________________________________________
Path of reaction
[5]
(c) Compounds with the same molecular formula but different structural
formulae are known as isomers. Complete these structural formulae to
show the three alkane isomers which have a molecular formula of C5H12.
C C
C C C C C C C C C C C C
C
pentane 2-methylbutane 2,2-dimethylpropane
[3]
(d) Suggest one physical method which could be used to distinguish
between these three isomers. __________________________________________
[1]
5. One radioactive isotope of cobalt, Co-60, has been used in the
treatment of cancer; it has a half-life of 5.3 years; and spontaneously
emits both b-particles and g-rays (i.e., high speed electrons and high
frequency photons, respectively). Determine how many protons, neutrons,
and electrons there are in each of the following.
Co-59 _________________________________________________________________
Co-60 _________________________________________________________________
Co(III)-60 ____________________________________________________________
[3]
METALS: TIN & LEAD
Tin, a rare element in the Earth's crust (0.0002%), occurs mainly in
the ore cassiterite [impure tin(IV) oxide]. The metal was obtained
first in the Bronze Age, when a mixture of coal and rocks was heated
in air; so, presumably, one of the reactions that occurred was:
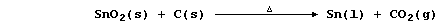
Modern technology uses coke and purified cassiterite in a furnace: but,
otherwise, the extractive method used to obtain tin, as well as copper
and lead, has not fundamentally changed over 7000 years.
[.. K > Ca > Mg > Al > Zn > Fe > Co > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. The principal ore of lead, a relatively rare element (0.001%) in
Earth's crust, is galena [impure lead(II) sulfide]. Lead(II) oxide is
formed when this ore is heated in air, and the metal is extracted by
the chemical reduction of this oxide with carbon in a furnace.
(a) Construct the symbol equation for each reaction in this process.
_______________________________________________________________________
_______________________________________________________________________
[4]
(b) Carbon and carbon monoxide are the commonest reducing agents used
industrially to reduce metal oxides, but others are also used (e.g.,
aluminium and dihydrogen). In the laboratory, reductions with hydrogen
gas can be attempted using the apparatus shown in this diagram.
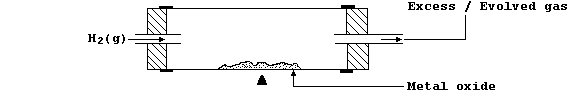
This is an open system in which equilibrium is never reached; thus, as
predicted by Le Chatelier's Principle, the stream of dihydrogen ensures
that the position of equilibrium continually 'shifts to the right'.
The Table below shows the calculated heat energy changes for the
reduction of various metal(II) oxides with dihydrogen; i.e.,
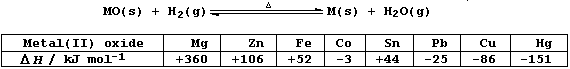
State and explain the effect of a temperature increase on the:
Rate of each reaction _________________________________________________
_______________________________________________________________________
_______________________________________________________________________
Yields of products in each endothermic reaction _______________________
_______________________________________________________________________
_______________________________________________________________________
Yields of products in each exothermic reaction ________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
2. Tin, (m.pt. = 232°C; r = 7.29 g cm-³), and lead, (m.pt. = 327°C; r
= 11.34 g cm-³), are both used extensively in alloys. Name two alloys
which contain either or both metals. __________________________________
[2]
3. Tin is used to manufacture 'tin-plate' (i.e., steel plated with a
layer of tin). Explain how tin protects steel from corrosion. _________
_______________________________________________________________________
_______________________________________________________________________
[2]
4. Approximately half of the lead produced is used in the manufacture
of storage batteries; in turn, these are recycled to provide about 40%
of industry's requirements of lead. A lead storage battery consists of
six cells connected in series; each 2 V cell, which has a lead anode, a
cathode made of lead(IV) oxide, and an electrolyte of aqueous sulfuric
acid, delivers high currents for short periods of time.
(a) When a battery discharges (i.e., when in use providing the required
voltage), the reactions occurring at the electrodes are: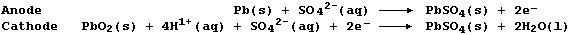
State the energy change which occurs during discharge. ________________
_______________________________________________________________________
[2]
(b) Suggest and explain one reason why such batteries are labelled with
the hazard symbol 'No smoking'. _______________________________________
_______________________________________________________________________
[2]
(c) Recharging the battery involves applying an external voltage to the
anode and cathode. What name is given to this reversal of the above
electrochemical reactions? ____________________________________________
[1]
5. Compounds of tin and lead have few (if any) beneficial functions in
living organisms. However, lead compounds are known to be particularly
toxic, because lead(II) ions inhibit the 'active sites' of a number of
enzymes; furthermore, as with so many non-biodegradable biocides, they
accumulate up the trophic levels.
(a) Name two sources of the lead ions introduced into the environment,
apart from lead storage batteries and mine-workings. __________________
_______________________________________________________________________
[2]
(b) The diagram below represents one food chain in the complex food web
of a typical slow-moving river in a temperate region.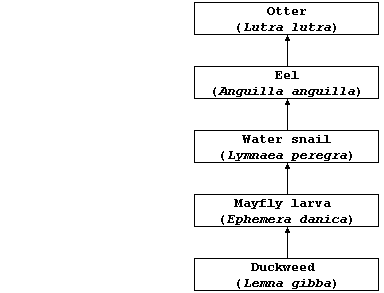
Estimate the amount of lead(II) ions which could be accumulated by one
otter, assuming: that each consumer in this food chain obtains its
chemical energy by eating 10 individuals of the organism at the trophic
level immediately below it; that none of the consumers egest or excrete
these ions; and that each duckweed plant absorbed 0.01 mg of these
ions. _________________________________________________________________
[2]
Name the source of energy for the producer in this food chain. ________
[1]
METALS: EXTRACTION of COPPER
Copper, which is relatively rare in the Earth's crust (0.006%), occurs
mainly as a sulfide (e.g., the ore chalcopyrite contains CuFeS2). This
metal, which has been shown to be essential to all biological species,
is a typical transition element. Thus, copper has a high melting point
(1083°C) and a high density (8.92 g cm-³), forms coloured compounds
(which are often blue or green), and shows variable oxidation states
[e.g., Cu(I) and Cu(II)]. Furthermore, copper and many of its compounds
show catalytic activity [e.g., copper(II) oxide is used as a catalyst
in the manufacture of methanol, and several copper-containing enzymes
are involved in photosynthesis and in respiration].
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. There are several routine methods for the extraction of copper, but
the processes used, which can be fairly complex, are dependent on both
the composition and quality of the ore. However, the final steps in one
method can be summarized by the following pair of equations:
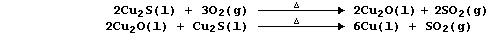
In industry, copper is electrolytically purified using slabs of impure
copper as anodes, thin sheets of pure copper as cathodes, and solutions
of copper(II) sulfate as electrolytes; this industrial process can be
modelled in the laboratory using the electrical circuit shown as Cell 1
in this diagram.
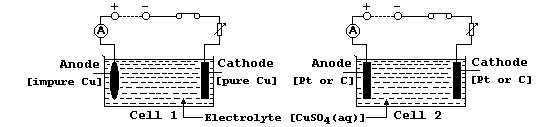
Aqueous copper(II) sulfate contains Cu2+(aq), SO42-(aq), OH1-(aq), and
H1+(aq) ions. When this solution is electrolyzed, the cathodic reaction
always results in copper being deposited, but the anodic reaction is
dependent on the conductor acting as the anode: aqueous copper(II) ions
are formed using a copper anode (Cell 1), whereas oxygen gas is evolved
using either a platinum or carbon-graphite anode (Cell 2).

(c) In cell 1, providing the anode is replaced periodically, continued
electrolysis results in the electrolyte remaining blue-coloured. In
cell 2, by contrast, continued electrolysis results in the electrolyte
becoming colourless [as the concentration of Cu2+(aq) ions decreases].
Suggest what this colourless solution contains. _______________________
[1]
2. Copper is used extensively in water piping, alloys, electroplating,
and in electrical wiring. Suggest two reasons for this widespread use.
_______________________________________________________________________
[2]
3. An industrial chemist investigated the following hypothesis: 'For
the electrolysis of aqueous copper(II) sulfate, the number of moles
(n) of copper deposited at a cathode increases in direct proportion to
time (t); i.e., n = k × t'; the Table shows a summary of the chosen
conditions and raw data.
Constants: aqueous copper(II) sulfate (600 cm³; 0.20 mol dm-³); direct
current (5.35 A); room temperature (17°C); surface area and distance
between copper electrodes (values measured but not recorded).
Time (t) / s |
0 |
60 |
120 |
180 |
180 |
180 |
240 |
300 |
Cu deposited / mg |
0 |
110 |
210 |
250 |
315 |
325 |
430 |
530 |
Cu deposited (n) / mmol |
0.0 |
1.7 |
3.3 |
3.9 |
4.9 |
5.1 |
6.7 |
8.3 |
(a) A value of a dependent variable which does not follow the general
pattern is usually referred to as an 'anomalous result'. In this
investigation, for example, the first value of n when t = 180 s appears
anomalous. Suggest one possible reason for this anomaly. ______________
_______________________________________________________________________
[1]
(b) Noting that the independent variable is on the horizontal axis,
plot all nine data points on this fully labelled graph paper, and then
draw a best straight line through as many points as is sensible.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Cu 8_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
d 7_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
p 6_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
o |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
s 5_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
i |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
t 4_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
d 3_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
(n) 2_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
/ 1_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
mmol 0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | |
0 60 120 180 240 300
Time (t) / s
[3]
This graph shows direct proportionality between the two variables,
because the straight line passes through the origin. Determine the
gradient of the graph; this value, 'k', is the proportionality constant
in the directly proportional relationship n = k × t.
(y2 - y1)
k = ————————— =
(x2 - x1)
[3]
constants). 'When a direct current of _______ was passed through an
electrolysis cell containing _______ electrodes and 600 cm³ of aqueous
copper(II) sulfate (0.20 mol dm-³), at room temperature (17°C), the
number of moles (n) of copper deposited at the cathode increased in
direct proportion to the time (t) of the electrolysis (within the
range __________); i.e., n = k × t, where k = ________________.'
[4]
METALS: DISPLACEMENT REACTIONS (2)
A displacement reaction is the chemical change which occurs when a more
reactive element displaces a less reactive element from its compound;
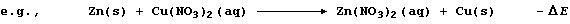
In this redox reaction, electrons are transferred from zinc atoms to
copper(II) ions because zinc is a better reducing agent (i.e., it is
more easily oxidized); the redox half equations are:
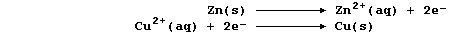
The aqueous nitrate ions are spectators, so the net ionic equation is:
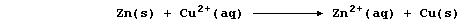
[.. K > Sr > Ca > Mg > Be > Al > Zn > Fe > Sn > (H) > Cu > Hg > Ag ..]
1. Temperature rises (DT) were measured for displacement reactions
involving beryllium and aqueous solutions of metal nitrates; this first
Table shows a summary of the chosen conditions and raw data.
Constants: volume (25 cm³), concentration (0.25 mol dm-³), and starting
temperature (17°C) of aqueous nitrate; beryllium powder (an excess);
thermometer (0-100°C); reaction vessel (an insulated plastic cup).
Nitrate(aq) |
Mg(II) |
Mg(II) |
Al(III) |
Zn(II) |
Pb(II) |
Cu(II) |
DT / °C |
0 |
0 |
6 |
14 |
32 |
45 |
(a) Beryllium compounds are extremely toxic. Apart from wearing safety
glasses, suggest one other vital safety precaution adopted for these
experiments. __________________________________________________________
[1]
(b) For the reaction between beryllium and aqueous copper(II) nitrate,
construct the symbol equation and state two other observable changes.
_______________________________________________________________________
_______________________________________________________________________
[3]
(c) State why no temperature rise was observed with aqueous magnesium
nitrate. ______________________________________________________________
[1]
(d) Use the results in this first Table to predict the temperature rise
(DT) that would be obtained, using the same set of constants, with
these aqueous nitrates: Sr(II) _______ Fe(II) _______ Ag(I) _______
[3]
2. A research chemist investigated the following hypothesis: 'The
speed (S) of reaction between beryllium and aqueous hydrochloric acid
increases in direct proportion to the concentration (C) of the acid;
i.e., S = k × C'; this second Table shows a summary of the chosen
conditions and raw data.
Constants: length (6.0 cm) and surface area (6.0 cm²) of beryllium
ribbon; volume of solution (25 cm³); room temperature (21°C); absence
of catalysts; volume of dihydrogen collected via gas syringe (25 cm³).
Concentration (C) / mol dm-³ |
0.0 |
0.0 |
0.15 |
0.30 |
0.45 |
0.60 |
0.75 |
Reaction time (t) / s |
»900 |
»900 |
120 |
58 |
42 |
32 |
25 |
Reaction speed (S) / ms-¹ |
0.0 |
0.0 |
|
|
|
|
40 |
(a) Construct the net ionic equation for the displacement reaction
investigated in the above hypothesis. _________________________________
_______________________________________________________________________
[2]
(b) Calculate the missing values for the reaction speed, and insert
these data in this second Table above.
[2]
(c) Label both axes, and then plot all seven data points.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
40_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
30_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
20_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_| Beryllium |_|_|_|_|_|_|_|_|_|_|_|
10_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | | |
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7
[4]
Draw a best straight line through as many points as is sensible, and
then determine the gradient of the (beryllium) graph; this value, 'k',
is the proportionality constant in the directly proportional
relationship S = k × C. _______________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Write a precisely worded conclusion based on the (beryllium) graph.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[5]
(d) Strictly speaking, to extrapolate a relationship outside the range
of the independent variable examined is scientifically flawed: but to
do so has practical advantages with respect to safety considerations,
time, and costs. Use the equation, and your value of 'k', to estimate
the reaction time if concentrated hydrochloric acid was used (i.e.,
C = 10 mol dm-³; pH -1). ______________________________________________
_______________________________________________________________________
[2]
(e) Sketch the graphs that would be obtained if Be was replaced with Ca
and with Ag. Recalling that there is no connection between the energy
change and the activation energy, suggest and explain using a symbol
equation, why each graph differs from the one obtained for Be.
Calcium _______________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
Silver ________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
METALS: DISPLACEMENT REACTIONS (3)
A catalyst is defined as 'a substance which increases the rate of a
chemical reaction, but which is itself chemically unchanged at the end
of the reaction'. Although many substances have catalytic activity
attributed to them, because they speed up chemical reactions, the
absence of permanent chemical change is often assumed ...
Zinc is a better reducing agent than hydrogen, and so displaces this
less reactive element from its compounds; e.g.,
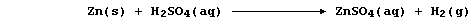
Because the above reaction is too slow to be useful as a convenient
laboratory preparation of dihydrogen, a few drops of aqueous copper(II)
sulfate are often added to increase the speed of reaction; and so the
preparative method is usually described by this equation:
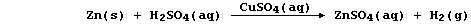
However, as the aqueous sulfate ions are spectators in this reaction
mixture, and as zinc is also a better reducing agent than copper, there
will be (at least) two competing reactions; i.e.,
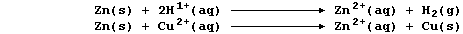
In principle, because copper(II) ions are better oxidizing agents than
hydrogen ions, zinc should preferentially displace copper; if this is
so, then the method might be better described by this equation:
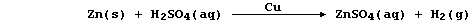
Although this description is supported by the presence of copper powder
at the end of the reaction, alternatives are feasible - particularly
when one considers the possible involvement of copper(I) ions.
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. One consequence of the above definition is that a catalyst has no
effect on the yield of products (i.e., the position of equilibrium in a
closed system). Although the laboratory preparation of hydrogen gas is
normally executed in an open system, the yield does appear to decrease
as the amount of copper(II) ions increases. To examine quantitatively
this effect, the following hypothesis was investigated: 'For the
reaction of zinc with dilute sulfuric acid, the volume (V) of hydrogen
gas evolved decreases in linear proportion to the amount (M) of aqueous
copper(II) ions added; i.e., V = k × M + c'; the Table shows a summary
of the chosen conditions and raw data (no duplicate experiments were
executed).
Constants: zinc granules (0.260 g = 4.00 mmol); aqueous sulfuric acid
(1.00 mol dm-³; 100 cm³); aqueous copper(II) sulfate (1.00 mol dm-³);
room temperature (298 K) and pressure (100 kPa); thermostatted water-
bath; hydrogen gas collected with a 100 cm³ gas syringe.
Amount of Cu2+ added (M) / mmol |
0.50 |
1.00 |
1.50 |
2.00 |
2.50 |
Volume of H2 evolved (V) / cm³ |
84 |
73 |
61 |
47 |
36 |
(a) Noting that hydrogen gas was evolved in these experiments, suggest
one important safety precaution adopted. ______________________________
[1]
(b) Suggest one reason why higher values of the independent variable
were not examined. ____________________________________________________
[1]
Explain what would happen to the values of the dependent variable if,
during the investigation, the temperature increased (but the pressure
remained constant). ___________________________________________________
_______________________________________________________________________
[2]
(c) Plot all five data points, and then draw the best straight line.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
V |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
o 90_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
l |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
u |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
m |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
70_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
o |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
f |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
gas |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
50_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
(V) |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
/ |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
cm³ 30_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
\_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0_/_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | |
0 0.5 1.0 1.5 2.0 2.5 3.0
Amount of aqueous Cu(II) added (M) / mmol
[3]
Extrapolate this straight line to the vertical axis; the intercept on
this axis, which corresponds to the maximum volume of hydrogen gas that
can be obtained, is the value 'c' in the linearly proportional
relationship V = k × M + c. ___________________________________________
[1]
Determine the gradient of the graph; this (negative) value, 'k', is the
proportionality constant in the linearly proportional relationship
V = k × M + c. ________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
Write a precisely worded conclusion based on the graph. _______________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[5]
(d) This linear relationship rearranges to M = (V - c) ÷ k. Determine
the amount of copper(II) ions which results in no hydrogen gas being
evolved (i.e., V = 0 cm³), by using your values of 'k' and 'c' in this
rearranged equation. __________________________________________________
[2]
(e) Experiments were also executed using different metals, but with a
similar set of constants; the aqueous copper(II) ions were omitted.
Thus, when 4.00 mmol of magnesium was used, instead of zinc, the same
maximum volume of hydrogen gas was obtained. Explain briefly what
theoretical volume of dihydrogen would be expected using 4.00 mmol of:
Cadmium _______________________________________________________________
Strontium _____________________________________________________________
Lithium _______________________________________________________________
Gallium _______________________________________________________________
[8]
METALS: PRECIPITATION REACTIONS
A precipitation reaction is the chemical change which occurs when two
ionic reactants give an insoluble product from an aqueous solution;
Because an ionic compound dissociates into (hydrated) cations and
anions when dissolved in water, a better perspective of a precipitation
reaction is gained by constructing an ionic equation; i.e.,
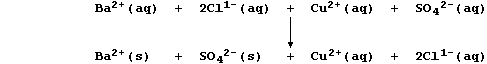
Those ions that are not involved in the overall reaction, here Cu2+(aq)
and Cl1-(aq), are referred to as spectator ions; and, so as to focus on
the change that actually occurs, a net ionic equation is often written;
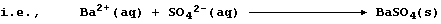
In order to be able to predict whether a precipitate will be formed
when two aqueous solutions are mixed, one needs to know the solubility;
i.e., the maximum amount of solute that can be dissolved in the solvent
water at a specific temperature. Extensive compilations of solubility
data are available in reference books (e.g., The Handbook of Physics
and Chemistry), though it is common practice to divide compounds into
three broad categories based on their solubilities in water at 20°C;
i.e., 'soluble' (> 10 g kg-¹), 'slightly soluble' (1 - 10 g kg-¹), and
'insoluble' (< 1 g kg-¹). These categories are then used to formulate
'solubility rules' - as exemplified by A ————®
A. All Group 1 and ammonium compounds are soluble.
B. All nitrates, hydrogencarbonates, and ethanoates are soluble.
C. Most chlorides, bromides, and iodides are soluble; the exceptions
are those containing silver(I) or lead(II) ions.
D. Most sulfates are soluble; CaSO4, SrSO4, and Ag2SO4 are slightly
soluble, whereas BaSO4 and PbSO4 are insoluble.
E. Most carbonates, phosphates, and sulfides are insoluble; the
exceptions are those containing Group 1 or ammonium ions.
F. Most hydroxides are insoluble; Ca(OH)2 and Sr(OH)2 are slightly
soluble, whereas Ba(OH)2 and Group 1 hydroxides are soluble.
1. Probably the most familiar example of a precipitation reaction is
that observed in the limewater test for carbon dioxide; the formation
of the characteristic milky-white precipitate is usually summarized as: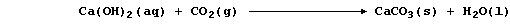
However, this symbol equation clearly requires explanation ... The gas
carbon dioxide, a covalently bonded compound, dissolves slightly in
water to form carbonic acid, H2CO3(aq), which partially dissociates;
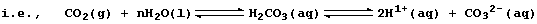
Le Chatelier's Principle predicts that both equilibria will shift to
the right if aqueous carbonate ions are removed from solution; and, as
the ionic equation below shows, this will occur by precipitation:
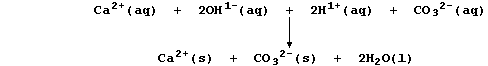
(a) This ionic equation reveals a precipitation and a neutralization
reaction. Construct a net ionic equation for each reaction. ___________
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) Continued bubbling of carbon dioxide through limewater results in
the (initially formed) precipitate redissolving. Construct the symbol
equation for this reaction. ___________________________________________
_______________________________________________________________________
[2]
2. The precipitation reaction between aqueous solutions of potassium
iodide and lead(II) nitrate was investigated quantitatively.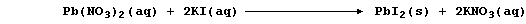
Using a pipette, 10.0 cm³ of aqueous lead(II) nitrate (0.100 mol dm-³)
was added to a test tube, and then, using a burette, 3.0 cm³ of an
aqueous solution of potassium iodide was added. The reaction mixture
was shaken thoroughly, and then, in the absence of a centrifuge,
allowed to stand for 1 hour before the height of the yellow precipitate
was measured (to within 0.5 mm). This procedure was repeated several
times, except different volumes of aqueous potassium iodide were used;
the Table shows a summary of the results.
Vol. KI(aq)/cm³ |
0.0 |
3.0 |
6.0 |
9.0 |
12.0 |
15.0 |
18.0 |
21.0 |
24.0 |
27.0 |
Ht. ppte./mm |
|
4.5 |
8.0 |
11.5 |
16.5 |
20.0 |
24.0 |
27.0 |
27.5 |
27.0 |
(a) Insert the missing value in the above Table, and then plot all ten
data points. _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
27_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
24_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
21_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
Height of 18_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
precipitate 15_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
/ 12_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
mm 9_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
6_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | | | | |
0 3 6 9 12 15 18 21 24 27
Volume of KI(aq) / cm³
[3]
(b) Draw the two best straight lines, and then, by intersecting these
lines, determine the minimum volume of aqueous potassium iodide
required to react completely with 10.0 cm³ of aqueous lead(II) nitrate
(0.100 mol dm-³). _____________________________________________________
[3]
(c) Use this value, and the symbol equation, to state the concentration
of the aqueous potassium iodide solution used in this investigation.
_______________________________________________________________________
[1]
3. Soluble lead(II) ions, which inhibit the active sites of a number
of enzymes, are absorbed by diffusion across semi-permeable membranes;
so, soluble lead(II) nitrate is obviously more toxic than any insoluble
lead(II) compound. Nevertheless, the accidental ingestion of aqueous
lead(II) nitrate is rarely fatal, in humans at least, because insoluble
lead(II) ions are precipitated from the reaction between this solution
and the hydrochloric acid present in gastric juice. Construct both the
symbol and net ionic equations for the precipitation reaction between
aqueous solutions of lead(II) nitrate and hydrochloric acid. __________
_______________________________________________________________________
_______________________________________________________________________
[3]
METALS: LIGAND-EXCHANGE REACTIONS
A ligand-exchange reaction is the chemical change which occurs when one
ligand is replaced by another; a ligand (L) is a molecule or an ion
(coordinatively) bonded to a central atom or ion. This type of reaction
is exemplified by reactions summarized by this general equation:
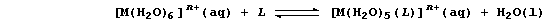
Hydrated metal ions, formed when an ionic compound dissolves in water,
can be represented either as Mn+(aq) or as [M(H2O)6]n+(aq) - because
each metal ion is usually coordinated to six water molecules acting as
ligands. The rates of ligand-exchange between coordinated and solvent
water molecules have been investigated using labelled water; i.e.,
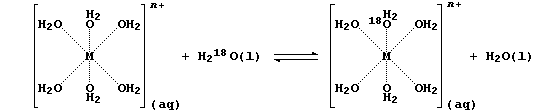
No attempt is made here to summarize the extensive research directed at
measuring and explaining the rates of these particular ligand-exchange
reactions; suffice to note that, as this order illustrates, .. Pb(II) >
K(I) > Cu(II) > Na(I) > Ca(II) > Zn(II) > Fe(II) > Mg(II) > Al(III) ..,
these rates do not correspond to the metal reactivity series.
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. Most molecules in living organisms are structurally very complex;
accordingly, in order to provide insight into some of their biological,
chemical, and physical properties, scientists often study simpler or
'model' compounds. Shown below are the structural formulae of two such
model compounds, which are readily prepared in two or three steps from
inexpensive reactants.
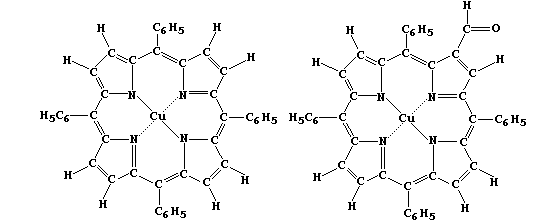
In each compound, the four nitrogens coordinated to the Cu(II) ion form
a square plane. Suggest the geometry of the compounds formed when:
A ligand is added above the plane. ____________________________________
Ligands are added above and below the plane. __________________________
[2]
Suggest what metal ion should replace Cu(II) in the above compounds for
either to be considered as a (marginally acceptable) model for studying
ligand-exchange reactions in:
Chlorophyll-b __________ Haemoglobin __________ Vitamin-B12 ___________
[3]
2. Probably the most familiar example of ligand-exchange reactions is
that observed in the standard test for the hydrated copper(II) ion with
(excess) aqueous ammonia; formation of the characteristic 'royal-blue'
solution is usually summarized as: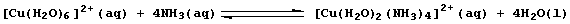
(a) Colour is an example of a qualitative variable; i.e., one where the
data are non-numerical or purely descriptive. In investigations, such a
variable can present problems in description (and, subsequently, in
interpretation), because of the inevitable element of personal opinion;
e.g., all ions corresponding to the formula [Cu(H2O)x(NH3)y]²+(aq) are
shades of blue, and the [Cu(H2O)2(NH3)4]²+(aq) ion has been variously
described as dark-blue, deep-blue, royal-blue, and indigo. Fortunately,
providing a suitable instrument is used, colour can be expressed as a
quantitative variable; i.e., one where the data are collected either by
counting or by measuring. Complete the Table below, by calculating the
frequency of the photon absorbed by each ion, using the formula n = fl,
where: n is the speed of light (3 × 108 ms-1); f is the frequency; and
l is the wavelength.
Aqueous ion Colour |
Wavelength of
photon absorbed
(l) / × 10-9 m |
Frequency of
photon absorbed
(f) / × 1014 Hz |
[Cu(H2O)6]2+ blue |
820 |
|
[Cu(H2O)5(NH3)]2+ ?-blue |
740 |
|
[Cu(H2O)4(NH3)2]2+ ?-blue |
675 |
|
[Cu(H2O)3(NH3)3]2+ ?-blue |
630 |
|
[Cu(H2O)2(NH3)4]2+ ?-blue |
585 |
|
[Cu(H2O)(NH3)5]2+ ?-blue |
635 |
|
(b) The symbol equation above summarizes a series of reversible ligand-
exchange reactions that involve the successive replacement of four
molecules of water with those of ammonia. Construct the symbol equation
for one of these reversible ligand-exchange reactions. ________________
_______________________________________________________________________
[2]
For a series of reversible reactions, using Le Chatelier's Principle to
predict the overall position of equilibrium is appropriate but not
straightforward. Usually, in investigating such series, the first step
is to measure various physical quantities; e.g., the concentrations of
reactants and products. Calculate the concentration of pure water. ____
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) In attempting to extend the scope of any given reaction, scientists
often consider variations (or develop hypotheses) based on the patterns
inherent in the Periodic Table; e.g., variants of Cu(II), H2O, and NH3
might reasonably include Ag(II), H2S, and PH3, respectively. An equally
rich source of variation lies in the patterns of organic chemistry ...
Each homologous series can be represented by a general formula; e.g.,
CnH2n+2 for chain alkanes. The so-called 'trivial' member of a series
is the compound whose formula corresponds to n = 0; this is dihydrogen
for the alkanes, whose first true member is methane. Several members of
an homologous series, which contains ammonia as the trivial member, act
as ligands in similar exchange reactions. Determine the general formula
of this series, and draw the structural formulae of its first two true
members. ______________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
METALS: DISPLACEMENT REACTIONS (4)
Pure water is a covalently bonded compound with some unusual physical
properties; these include a high boiling point, a high surface tension,
a maximum density as a liquid, a high latent heat of vaporization, and
the highest heat capacity of any liquid. All of these properties are
partially attributable to water's extended structure, which involves
'hydrogen bonding'; this type of bonding is present in many molecules,
(for example, and in particular, between the strands of DNA double
helices), but its extent in pure water appears to be truly exceptional.
However, pure water is rarely encountered in Nature because it is such
an excellent solvent in which many solutes dissolve to varying degrees.
Furthermore, the resulting aqueous solutions might quite reasonably be
expected to have different properties; e.g., heat capacities ...
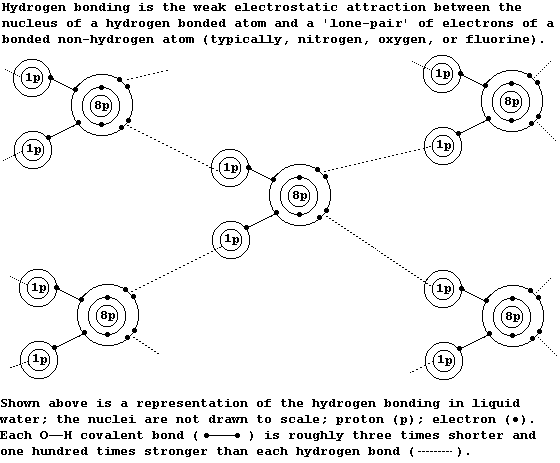
1. A research chemist decided to use a metal displacement reaction,
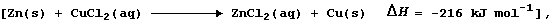
to examine this hypothesis: 'As the concentration (C) of chloride ions
increases, the specific heat capacities (c) of the aqueous solutions
decrease in linear proportion; i.e., c = k × C + i'; the Table shows a
summary of the chosen reaction conditions and raw data.
Constants: heat energy supplied (H = 0.540 kJ); powdered zinc (excess);
aqueous copper(II) chloride (0.25 mol dm-³; 10.0 cm³ via bulb pipette);
starting temperature of reaction mixture (20.0°C ± 0.2°C); thermometer
(0-50°C, graduated to 0.1°C); reaction vessel (thermos flask).
NaCl(s)
added /
g |
Concentration
of Cl1- (C) /
mol dm-³ |
Density
r /
kg dm-³ |
Total mass
m /
kg |
Temperature
rise (DT) /
K |
Specific heat
capacity (c) /
kJ kg-¹ K-¹ |
0.00 |
0.50 |
1.02 |
0.0102 |
13.8 |
|
0.59 |
1.50 |
1.05 |
0.0105 |
14.9 |
|
1.17 |
2.50 |
1.08 |
0.0108 |
14.8 |
3.38 |
1.17 |
2.50 |
1.08 |
0.0108 |
15.3 |
3.27 |
1.17 |
2.50 |
1.08 |
0.0108 |
15.5 |
3.25 |
1.76 |
3.50 |
1.11 |
0.0110 |
15.7 |
|
2.34 |
4.50 |
1.14 |
0.0113 |
15.7 |
|
(a) The standard equation H = m × c × DT rearranges to c = H ÷ m × DT.
Using this rearranged equation, calculate the missing values for the
specific heat capacity (c), and insert these data in the Table.
[3]
(b) The first specific heat capacity for C = 2.50 mol dm-³ appears to
be an anomalous result. Suggest one possible reason for this anomaly.
_______________________________________________________________________
[1]
(c) Label both axes, plot all seven data points, and then draw a best
curve through as many points as is sensible.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
3.8_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3.6_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3.4_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3.2_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3.0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0.0_/_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | | | | |
0.0 1.0 2.0 3.0 4.0
[5]
Complete this precisely worded conclusion, based on the tabulated and
graphical data. 'As determined from displacement reactions at room
temperature (20°C), using excess zinc powder and 10.0 cm³ of aqueous
copper(II) chloride (0.25 mol dm-³), specific heat capacities (c) of
aqueous solutions of sodium chloride decreased as the concentration (C)
of chloride ions __________ (within the range ___________________). The
curve indicates that these two variables are not in ___________________
to each other; i.e., ________________'
[4]
(d) Here, unfortunately, values of the dependent variable outside the
range of the independent variable examined cannot be obtained either
by calculation, because of the non-linear relationship between the
variables, or by extrapolation, because of the graph's limited range.
Nevertheless, from inspection of the tabulated data, guesstimate a
value for the specific heat capacity (c) of saturated aqueous sodium
chloride (C = 6.5 mol dm-³). __________________________________________
[1]
(e) Complete the following paragraph, which incorporates one partial
interpretation and one possible consequence of the results of the above
investigation. 'The specific heat capacity of an aqueous solution
decreases as the concentration of chloride ions __________, presumably
because the amount of hydrogen bonding __________. In consequence, its
ability to act as a heat buffer, in intra-cellular or extra-cellular
environments, will _________; furthermore, because all biochemical
reactions are controlled by temperature-sensitive enzymes, this may
result in __________ efficiency of these biological catalysts.'
[4]
METALS: SODIUM COMPOUNDS
The commercial applications of sodium compounds in developed countries
are so numerous that any brief overview has obvious limitations. But,
as a starting point, it does appear appropriate to state that their
extensive use is attributable to the following four aspects. Firstly,
sodium compounds are cheap to manufacture; in part, this reflects the
abundance and accessibility of the raw materials (e.g., sea water and
rock salt both contain sodium chloride). Secondly, sodium compounds are
usually soluble in water and have high thermal stability. Thirdly, in
most applications, it is the chemical properties of the anions which
are important; the sodium cations are usually mere spectators. And
fourthly, sodium cations are (relatively) non-toxic.
1. Brine is a concentrated aqueous solution of sodium chloride. Three
useful products are obtained from the electrolysis of brine, as this
overall symbol equation shows:
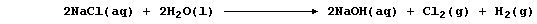
The ionic equations for the reactions occurring at the electrodes are:
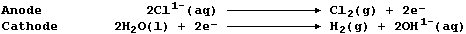
(a) Label this diagram of a (Diaphragm) electrolytic cell with: Cl2(g);
H2(g); NaOH(aq); Saturated brine; Steel cathode; and, Titanium anode.
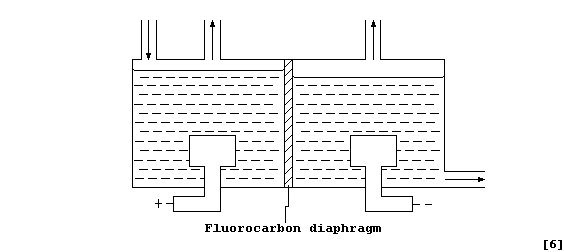
(b) A typical electrolytic cell used in industry, which operates
continuously at 1.5 kA and 4 V, must have at least five hazard symbols
clearly visible: 'Corrosive' [i.e., NaOH(aq)]; 'Explosive' [H2(g)];
'Highly flammable' [H2(g)]; 'Oxidizing' [Cl2(g)]; and 'Toxic' [Cl2(g].
State three items of safety clothing necessary for workers or visitors.
_______________________________________________________________________
[3]
(c) The diaphragm is selectively permeable, allowing hydroxide (but not
chloride) ions to enter the cathodic compartment. Suggest and explain
one advantage in this selectivity. ____________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
The diaphragm also prevents mixing of the gaseous electrode products,
dihydrogen and dichlorine, which together react explosively. Construct
the symbol equation for this reaction. ________________________________
_______________________________________________________________________
[1]
(d) Suggest and explain one reason why electrodes made of iron would be
unsuitable. ___________________________________________________________
_______________________________________________________________________
[2]
2. Aqueous sodium carbonate neutralizes aqueous hydrochloric acid:
As this ionic equation shows, it is the carbonate (and hydrogen) ions
which are the reactants: the sodium (and chloride) ions are spectators.
Construct a similar ionic equation for the precipitation reaction
between aqueous solutions of sodium hydroxide and iron(II) sulfate.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
3. Sodium carbonate is thermally very stable; indeed, it melts before
it decomposes at temperatures above 1000°C: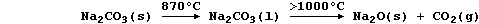
By contrast, sodium hydrogencarbonate is thermally much less stable;
thus, at temperatures above 90°C, it decomposes to give three products.
Construct the symbol equation for this thermal decomposition. _________
_______________________________________________________________________
_______________________________________________________________________
[2]
4. The Table below presents one illustrative application for eight
sodium compounds. Complete this Table by inserting the correct formula
from this list: [CH3(CH2)16COO]Na ; NaCl ; NaHCO3 ; NaNH2 ; NaOCl ;
NaOH ; Na2[B2(O2)2(OH)4] ; Na2CO3.
Formula of compound
(Application) |
Property of compound or anion which
is important in specified application |
(Strong base) |
Completely dissociates in water; the hydroxide
ions neutralize aqueous hydrogen ions. |
(Baking powder) |
Partially decomposes when heated; the evolved
carbon dioxide expands, so dough mixtures rise. |
(Fertilizer) |
Hydrolyzes to give ammonia, which is converted
to ammonium ions in the soil: so crops are
provided with a usable source of nitrogen. |
(Glass manufacture) |
Completely decomposes when heated at very high
temperatures; the resulting molten sodium oxide
fuses with other oxides to form glass. |
(Food preservative) |
A concentrated solution, which is non-toxic,
has a low water potential: so water is removed
from pathogenic cells by exo-osmosis across the
semi-permeable membranes. |
(Soap) |
Hydrocarbon chain is lipophilic and ions are
hydrophilic: so has detergent properties, as it
acts as an emulsifying agent for fats and oils. |
(Bleaching agent) |
Partially decomposes when heated to ca. 80°C;
the evolved hydrogen peroxide acts as a bleach. |
(Disinfectant) |
An aqueous solution slowly photolyzes to give
'chlorine water', which is a biocide. |
[8]
5. In Man, sodium ions are required principally for osmoregulation and
for conduction of nerve impulses. State briefly how excess sodium ions
are excreted in this species. _________________________________________
_______________________________________________________________________
[2]
6. Name the only other metal whose compounds are usually non-toxic,
water-soluble, thermally-stable, and cheap to manufacture. ____________
[1]
METALS: ALUMINIUM & ZINC COMPOUNDS
Both aluminium and zinc are used extensively in industry, either as the
pure metals or in alloys. By contrast, compounds of these metals have
found considerably fewer commercial applications, but some are used;
e.g., as rodenticides, in paint pigments, as fire retardents, and in
water purification [zinc phosphide, zinc oxide, aluminium hydroxide,
and aluminium sulfate, respectively.]
Aluminium and zinc form compounds with the metal ions in different
oxidation states [Al(III) and Zn(II)]. This difference may partially
explain why enzymes containing zinc - but not aluminium - are essential
to all biological species; particularly ubiquitous are the carbonic
anhydrases and the carboxypeptidases, which are involved in respiration
and protein hydrolysis, respectively. Nevertheless, clearly reflecting
their near-adjacent positions in the reactivity series, the chemistry
of these metals and their compounds show noteworthy similarities - as
these schemes perhaps over-emphasize?
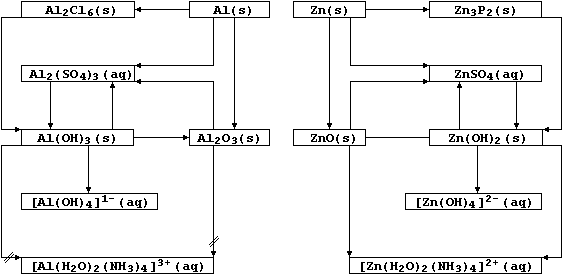
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. Aluminium and zinc are both used to test for the presence of
nitrate ions. Thus, heating an alkaline solution containing nitrate
ions with either powdered aluminium or zinc results in the evolution of
an alkaline gas (ammonia); the symbol equations for such reactions are:
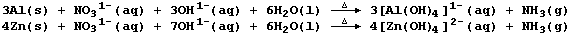
Suggest why the nitrate ion is considered to be reduced in this test.
_______________________________________________________________________
[1]
2. Aluminium chloride and zinc phosphide are both prepared directly by
heating their respective elements. Construct the symbol equation for
the synthesis of each of these covalently bonded compounds. ___________
_______________________________________________________________________
_______________________________________________________________________
[2]
3. Zinc phosphide hydrolyzes in water to give the extremely toxic gas
phosphine: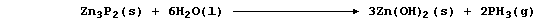
A number of aluminium compounds are hydrolyzed in a similar manner to
zinc phosphide. Predict the name and formula of the gas or solution
produced when each of the following are added separately to water.
Aluminium chloride (Al2Cl6) ___________________________________________
Aluminium nitride (AlN) _______________________________________________
Aluminium carbide (Al4C3) _____________________________________________
[6]
4. Zinc oxide is thermochromic; i.e., it changes colour with changes
in temperature: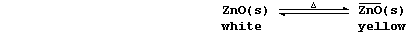
Suggest one reason why both processes should be considered as chemical
changes. ______________________________________________________________
[1]
5. Flammable polymers, such as poly(ethene) and poly(chloroethene),
are used extensively in furnishings; to reduce their potential as fire
hazards, flame retardents are often added to these materials. Construct
the symbol equation, complete with a qualitative indication of the heat
energy change, for the thermal decomposition of aluminium hydroxide.
_______________________________________________________________________
[2]
Suggest one way aluminium hydroxide acts as a fire retardent. _________
_______________________________________________________________________
[1]
6. As the reaction schemes show, the oxides and hydroxides of zinc and
aluminium react with both acids and bases; i.e., they are amphoteric
(cf. amphibians). Typical reactions which exemplify the amphoteric
character of aluminium oxide are summarized by this pair of equations: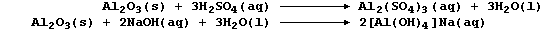
Complete the following symbol equations, which will summarize reactions
exemplifying the similarly amphoteric character of zinc oxide:
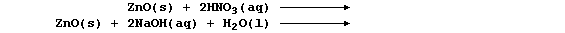
7. Aluminium hydroxide is used as a coagulating agent in helping to
remove suspended solids from domestic water supplies. Thus, the careful
addition of calcium hydroxide to tanks of water containing aluminium
sulfate results in the formation of aluminium hydroxide - which, as a
gelatinous precipitate, is ideal for trapping suspended solids:
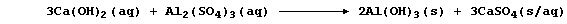
This symbol equation reveals two precipitation reactions. Construct a
net ionic equation for each of these reactions. _______________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Suggest one reason why the quantity of calcium hydroxide must be very
carefully controlled, apart from preventing the aluminium hydroxide
redissolving. _________________________________________________________
_______________________________________________________________________
[1]
In view of a possible connection between aluminium ions and Alzheimer's
disease (which causes premature senile dementia), suggest one compound
which might be a suitable alternative to aluminium hydroxide as the
coagulating agent. ____________________________________________________
[1]
8. Aqueous ammonia is one reagent used to distinguish between aqueous
solutions of aluminium and zinc ions. Thus, the dropwise addition of
aqueous ammonia to a solution containing aluminium ions results in the
formation of a white precipitate, Al(OH)3, which does not redissolve
in excess reagent. However, although the dropwise addition of aqueous
ammonia to a solution containing zinc ions similarly results in the
formation of a white precipitate, Zn(OH)2, this does redissolve in
excess reagent to form a solution containing a soluble tetraamminezinc
ion, [Zn(H2O)2(NH3)4]2+(aq). Suggest one method of distinguishing
between solid samples of aluminium oxide and zinc oxide, apart from
their reactions with aqueous ammonia. _________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
METALS: IRON COMPOUNDS
Compounds of iron range in complexity from haemoglobin, whose structure
is similar to both chlorophyll-a and vitamin-B12, to the examples shown
in the reaction scheme.
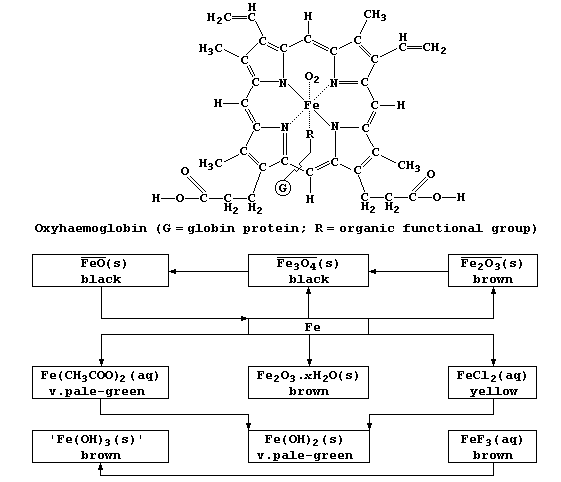
1. 1. Red blood cells contain the respiratory pigment haemoglobin, Hb.
In the absence of oxygen, a water molecule bonds to the iron(II) ion
to form deoxyhaemoglobin, HbH2O. Dioxygen reacts reversibly with HbH2O
to form oxyhaemoglobin, HbO2; this ligand-exchange reaction, which
involves an oxygen molecule replacing the water molecule and bonding
to the iron(II) ion, can be summarized in part by this equation:
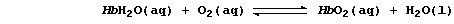
Carbon monoxide reacts reversibly with HbH2O, but irreversibly with
HbO2, to form carboxyhaemoglobin, HbCO; i.e.,
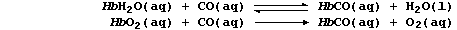
These three forms of haemoglobin colour the blood: dark-blue, HbH2O;
bright-red, HbO2; and cherry-red, HbCO. Cells which receive HbCO cannot
carry out aerobic respiration, and so cannot release sufficient ATP for
vital metabolic processes. Name two organs which contain cells that are
most likely to be affected by HbCO. ___________________________________
[2]
2. One method of preventing iron from forming rust, Fe2O3.xH2O(s), is
'to paint' the iron with a solution of phosphoric acid, H3PO4(aq); the
resulting layer of insoluble iron(III) phosphate provides the iron with
a physical barrier to water and dioxygen. Construct the symbol equation
for the reaction between iron and aqueous phosphoric acid. ____________
_______________________________________________________________________
3. Except for the precipitation reactions, leading to the formation of
iron(II) hydroxide and iron(III) hydroxide, explain carefully why all
the other reactions in the scheme are considered to be redox reactions.
_______________________________________________________________________
_______________________________________________________________________
[3]
4. Using a starting material shown in the scheme, construct the symbol
equation for one synthesis of:
Iron(II) ethanoate(aq) ________________________________________________
_______________________________________________________________________
Iron(III) fluoride(aq) ________________________________________________
_______________________________________________________________________
Iron(III) hydroxide(s) * ______________________________________________
_______________________________________________________________________
[6]
5. State and explain, using ionic equations, the observations at the
anode, at the cathode, and for the electrolyte when an aqueous solution
of iron(II) chloride is electrolyzed [the ions H1+(aq) and OH1-(aq),
though present, are not involved in the electrolysis].
Anode _________________________________________________________________
_______________________________________________________________________
Cathode _______________________________________________________________
_______________________________________________________________________
Electrolyte ___________________________________________________________
_______________________________________________________________________
[8]
6. Heterotrophs obtain their chemical energy via the hydrolysis of
organic compounds (e.g., carbohydrates, proteins, fats, and oils): by
contrast, autotrophs biosynthesize their chemical energy from inorganic
compounds (such as CO2, H2O, and H2S). The vast majority of autotrophs
are photosynthetic: but a significant minority are chemosynthetic. For
example, the bacteria Thiobacillus ferrooxidans obtain their source of
energy from the oxidation of iron(II) sulfide; the complex metabolic
processes involved can be loosely summarized by the following equation: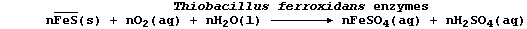
One of the metabolic by-products is dilute sulfuric acid. The passage
of this acid over ores containing insoluble copper(II) sulfide (CuS)
results in the formation of soluble copper(II) sulfate, which collects
in blue pools. Copper metal is extracted from these pools either by
addition of scrap iron or by electrolysis.
(a) Construct the symbol equation for the reaction between:
Aqueous sulfuric acid and copper(II) sulfide __________________________
_______________________________________________________________________
Iron and aqueous copper(II) sulfate ___________________________________
_______________________________________________________________________
[4]
(b) This extractive method for copper uses micro-organisms, albeit
indirectly, and so is an example of biotechnology; i.e., the industrial
use of genes from micro-organisms to obtain products considered useful
to Man. Genetic engineering, more correctly known as recombinant DNA
technology, maybe used to alter the genes of Thiobacillus ferrooxidans.
Suggest two possible applications for such altered bacteria. __________
_______________________________________________________________________
_______________________________________________________________________
[2]
* The currently accepted view is that the formula 'Fe(OH)3' is little
more than a convenient representation for various hydrated iron(III)
oxides; these substances are, incidentally, major constituents of soils
(and, as such, may be the commonest transition metal compounds observed
in everyday-life).
METALS: COPPER COMPOUNDS (1)
Most reactions of transition metal compounds can be usefully classified
into two types. First, those known as ligand-exchange reactions, which
involve the replacement of one or more ligands (coordinatively) bonded
to a central metal atom or ion; e.g.,

And second, those known as redox reactions, which involve changes in
oxidation states; e.g.,
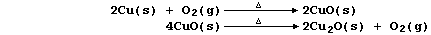
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. In a crystalline lattice there are strong electrostatic forces of
attraction between oppositely charged ions; these bonds are broken when
an ionic solute dissolves in the solvent water, and new ones are formed
in the aqueous solution (i.e., those between water molecules and ions).
Nonetheless, dissolution is usually presented as a simple physical
change ...
(a) Anhydrous copper(II) sulfate, CuSO4(s), is an off-white solid; it
dissolves in water to form aqueous copper(II) sulfate, CuSO4(aq),
which is a blue solution:
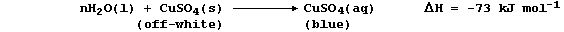
Suggest and explain one reason why this dissolution should be regarded
as a chemical change. _________________________________________________
_______________________________________________________________________
[2]
(b) Aqueous copper(II) sulfate is a mixture of Cu2+(aq), SO42-(aq),
H1+(aq), and OH1+(aq) ions. State the formulae of the two ions that are
present in (anhydrous) copper(II) sulfate, but not in this mixture.
_______________________________________________________________________
[2]
(c) Strong heating of aqueous copper(II) sulfate, in a distillation
apparatus, results in the quantitative recovery of copper(II) sulfate,
as an off-white solid, and water, as a colourless liquid: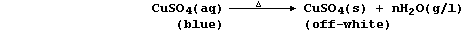
By contrast, slow evaporation of aqueous copper(II) sulfate results in
the formation of hydrated copper(II) sulfate as blue crystals:

State why both of these evaporative processes, nominally designated as
'physical', should be regarded as chemical changes. ___________________
_______________________________________________________________________
[1]
Name one dehydrating agent which can effect the dehydration of hydrated
copper(II) sulfate. ___________________________________________________
[1]
2. Copper, a poorer reducing agent than hydrogen, is not oxidized by
H1+(aq): so, in contrast to the metals above hydrogen in the reactivity
series, copper does not react with water, steam, or dilute acids; i.e.,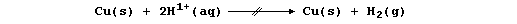
Nevertheless, copper can be oxidized by both concentrated sulfuric and
nitric acids; the oxidizing agents are, respectively, SO42-(aq) and
NO31-(aq). Construct a symbol equation for the oxidation of copper by:
Concentrated sulfuric acid (Mr of the gaseous product is 64). ________
_______________________________________________________________________
Concentrated nitric acid (Mr of the gaseous product is 46). ___________
_______________________________________________________________________
[4]
3. Copper(II) oxide can be reduced to copper by a number of reducing
agents, including more reactive elements; e.g.,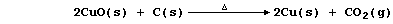
Construct a symbol equation for the reduction of copper(II) oxide with:
Ammonia (molar masses of the gaseous products are 28 and 18 g mol-¹).
_______________________________________________________________________
Propane (molar masses of the gaseous products are 44 and 18 g mol-¹).
_______________________________________________________________________
[4]
4. Probably the most familiar example of a copper(I) compound is that
observed when a positive result is obtained in the Fehling's test for
'reducing sugars'; e.g., warming an alkaline solution of glucose and
copper(II) sulfate results in the precipitation of copper(I) oxide as
a pale-orange solid. Suggest why a carbohydrate which gives a positive
result in this test is known as a reducing sugar. _____________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Name one other reducing sugar. ________________________________________
[1]
5. Aqueous solutions of copper(I) ions are unstable with respect to
aqueous copper(II) ions and copper; i.e.,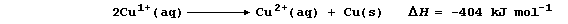
A blue solution and a brown precipitate are formed when copper(I) oxide
is warmed with dilute sulfuric acid. Construct the symbol equation for
this redox reaction. __________________________________________________
_______________________________________________________________________
[2]
6. Ethene is the simplest plant growth regulator known; it stimulates
the lateral expansion of elongating cells and promotes fruit ripening
and leaf drop. Researchers have synthesized several copper(I) compounds
which appear to be reasonably suitable models for the 'binding site' of
ethene; shown below is the structural formula of a typical example. [In
animals, the nearest equivalent of a binding site is the receptor site
associated with a chemical synapse (where neurotransmitters such as
acetylcholine and dopamine are released)].
Using patterns inherent in the Periodic Table and/or organic chemistry,
draw the structural formulae of two copper(I) analogues of the above
model compound; for clarity, omit the anions. _________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
METALS: COPPER COMPOUNDS (2)
Metal ions are regarded as pollutants when, as a direct or indirect
result of Man's activities, their concentrations in any given ecosystem
increase. Popular attention invariably focuses on the toxic effects of
ions which have little or no biological rôle; e.g., those of aluminium,
cadmium, lead, and mercury. However, a correctly balanced perspective
of pollution also requires an awareness of the potential toxicity of
biologically essential ions; e.g., those of copper, iron, manganese,
and zinc. Thus, although each species has evolved mechanisms of using,
and adapting to, molecules and ions at their ambient concentrations,
adverse effects occur at higher concentrations because such homeostatic
mechanisms are overwhelmed. Increased concentrations of hydrated metal
ions in the environment are most commonly attributable to: the use of
biocides; leaching from active or derelict mines; the illegal discharge
of industrial waste; and, the products of neutralization reactions
between the components of 'acid rain' and metal ores.
1. Various copper(II) compounds, including the ethanoate, hydroxide,
and sulfate, have been used as fungicides and as insecticides; this
scheme summarizes their syntheses via neutralization and precipitation
reactions.
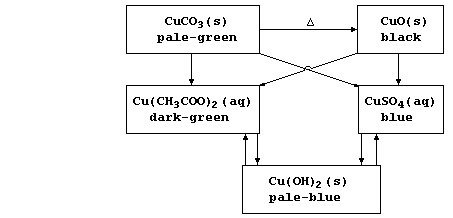
(a) Construct the symbol equation for the neutralization of:
Dilute ethanoic acid with copper(II) carbonate ________________________
_______________________________________________________________________
Dilute sulfuric acid with copper(II) oxide ____________________________
_______________________________________________________________________
[2]
Complete the following description of one method of preparing crystals
of copper(II) sulfate pentahydrate. "Wear safety glasses. To 25 cm³ of
gently-boiling dilute sulfuric acid, add copper(II) oxide in small
portions until the reaction mixture no longer turns universal indicator
paper ____. Filter this mixture, _________ evaporate the filtrate using
a boiling water-bath, and then leave the blue solution to _____________
at room temperature in a labelled petri dish covered with paper."
[3]
(b) Construct the net ionic equation for the precipitation reaction
summarized by this symbol equation: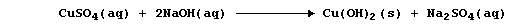
_______________________________________________________________________
[1]
Complete the following description of one method of preparing a dry
sample of copper(II) hydroxide. "Wear safety glasses and _______. Use a
burette to add 5.0 cm³ of aqueous copper(II) sulfate (1.00 mol dm-³)
into a test-tube. Use a separate burette to add carefully _________ of
aqueous sodium hydroxide (1.00 mol dm-³) to the blue solution in the
test-tube. Shake the mixture thoroughly, centrifuge, and then decant
the colourless supernatant. Add about 15 cm³ of __________ water to the
precipitate, and then repeat the shaking, centrifuging, and decanting
steps. Leave the precipitate to dry in a labelled petri dish."
[3]
2. A number of grass species, including Agrostis tenuis, have evolved
tolerance to certain metal ions; e.g., those of copper, lead, and zinc.
A partial explanation of the evolution of a grass species tolerant to
high concentrations of copper(II) ions is included below.
(a) A mutation is the spontaneous change in the structure of DNA or of
RNA, involving either a whole chromosome or an allele (i.e., a sequence
of nucleotides coding for one polypeptide). Mutations occur naturally
all the time, but various types of radiation (and chemicals) increase
their frequency. Name one type of radiation which increases mutation
rates. ________________________________________________________________
[1]
(b) A species adapts to ambient concentrations of copper(II) ions, and
so homozygous recessive (tt) parents will produce offspring which
show no tolerance to excess copper(II) ions. However, when a favourable
mutation occurs in the reproductive cells of one grass plant, resulting
in the formation of a dominant allele (T) that codes for tolerance,
then its genotype becomes Tt and its phenotype 'tolerant'. As this
first genetic diagram shows, 50% of the offspring produced by this
heterozygous (Tt) parent and a homozygous recessive (tt) parent will be
'tolerant'; these will be the fittest in an environment where the agent
of selection is a high concentration of copper(II) ions, and so be the
most likely to survive to reproductive maturity.
Construct a second genetic diagram to show the genotypes and phenotypes
of the offspring produced by parents who are both heterozygous. _______
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
State the genotype and phenotype of all the offspring produced by a
homozygous dominant (TT) parent who reproduces by self-fertilization.
_______________________________________________________________________
[2]
(c) Tolerant grass species are now used in industrial regions to turn
derelict mines and slag heaps into recreational areas. Recalling that
these autotrophs are the producers in diverse food chains, suggest and
explain one possible ecological disadvantage of this attractive method
of conservation. ______________________________________________________
_______________________________________________________________________
[2]
METALS: MERCURY
Mercury, a very rare element in the Earth's crust (0.000008%), occurs
mainly in ores either as a sulfide or native. This metal, which has a
low melting point (-39°C) and a low boiling point (357°C), is a mobile
liquid at room temperature. Mercury's high density (13.6 g cm-³),
uniform coefficient of expansion, electrical conductivity and fluidity,
high volatility, and ability to dissolve many metals, has resulted in
its widespread use (e.g., in thermometers, as electrical contacts, in
vapour lamps, and in the formation of alloys known as amalgams).
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. Mercury is usually extracted by heating mercury(II) sulfide in air
to form mercury(II) oxide, followed by thermal decomposition of this
oxide to give mercury vapour (which is then condensed). Construct the
symbol equation for each reaction in this extraction process. _________
_______________________________________________________________________
_______________________________________________________________________
[4]
2. Mercury vapour is very dangerous because it destroys lung and brain
tissues. Suggest and explain one reason why areas affected by small
spillages of mercury are immediately covered with powdered sulfur. ____
_______________________________________________________________________
_______________________________________________________________________
[2]
3. The ingestion of small quantities of elemental mercury from dental
amalgams appears to result in no harmful effects. State and explain the
reason why mercury ions are not formed from mercury and the aqueous
hydrochloric acid present in gastric juice. ___________________________
_______________________________________________________________________
[2]
4. Presented here are partial explanations for the toxicity of, and
the tolerance shown towards, mercury compounds in living organisms ...
Proteins are formed by condensation reactions between carboxylic acid
(-COOH) and amino (-NH2) functional groups of amino acids. One of these
amino acids is known as cysteine, which contains a thiol (-SH) group.
Frequently, the thiol groups on the polypeptide chains react together
to form 'disulfide bridges' (i.e., as shown in "A"). These bridges are
important in determining the three-dimensional shapes of proteins and,
therefore, their correct biological function.
Mercury compounds react with these disulfide bridges to form proteins
containing inserted mercury (i.e., as shown in "B"); these insertions
change their (optimal) three-dimensional shapes, and so toxic effects
often result. By way of contrast, many living organisms have evolved a
limited degree of tolerance towards such compounds by biosynthesizing
'cysteine-rich' proteins, whose polypeptide chains contain numerous
thiol groups; these react similarly with mercury ions, and so act as an
effective homeostatic mechanism of detoxification.
(a) Name the group of catalytic proteins whose efficacy might decrease
as a result of mercury insertions into disulfide bridges. ____________
[1]
(b) Name another element whose ions might react with the thiol groups
of cysteine-rich proteins. ____________________________________________
[1]
5. Compounds of mercury are used, often indiscriminately, as biocides:
unfortunately, in common with those of a number of other heavy metals,
they are accumulated up the trophic levels. This diagram represents a
simple food chain in the complex Antarctic food web.
(a) The protoctistans Navicula perpusilla, apart from being the major
producers of chemical energy in marine food webs, provide a huge amount
of the biosphere's supply of dioxygen. By what light-dependent process
do these protoctistans produce this gas? ______________________________
[1]
(b) The crustaceans Euphausia superba, commonly known as 'krill', build
their exoskeletons by incorporating which metal ions? _________________
[1]
(c) Estimate the amount of mercury(II) ions which could be accumulated
by one blue whale (Sibaldus musculus), assuming that each protoctistan
absorbed 2 x 10-¹³ g of mercury(II) ions and that none of the consumers
egest or excrete these ions. __________________________________________
_______________________________________________________________________
[2]
6. Certain anaerobic bacteria have evolved an ability to metabolize
elemental mercury, which is present in the environment largely because
of the illegal discharge of industrial waste. These organisms excrete
several toxic metabolites, including dimethylmercury; the biosynthesis
of this (lipid-soluble) compound involves vitamin-B12.
(a) Mercury's ability to form amalgams is utilized in the extraction of
precious metals - often in areas rightly considered as non-renewable
natural resources (e.g., rainforests). Name one precious metal which is
extracted via amalgamation with mercury. ______________________________
[1]
(b) Dimethylmercury thermally decomposes to give ethane and mercury;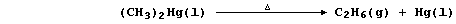
The equation for this thermal decomposition can be written as follows:
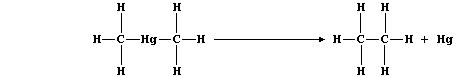
Complete the Table below using these bond energies (in kJ mol-¹):
C-H (413); C-Hg (109); and C-C (346).
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
|
|
|
|
|
|
|
|
Total = |
Total = |
[3]
Calculate the heat energy change (DH) for the above decomposition. ____
_______________________________________________________________________
[2]
(c) Hitherto, the metabolic activities of these bacteria have focused
attention on the implications for various ecosystems. Nevertheless, one
should also be aware that their use in a fermentation process could
provide an opportunity for developing a renewable energy source (i.e.,
ethane). Construct the symbol equation for the combustion of ethane.
_______________________________________________________________________
[2]
METALS: SILVER
Silver, which is extremely rare in the Earth's crust (0.000007%), is
usually found in ores either as a sulfide or native. This element is a
typical transition metal, as evinced by its (fairly) high melting point
(962°C), high density (10.49 g cm-³), variable oxidation states [e.g.,
(colourless) Ag(I) and (coloured) Ag(II)], and catalytic activity
(e.g., it is used in the oxidation of ethene to ethane-1,2-diol).
[.. K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. One of several methods of extracting silver from low-grade ores
involves heating silver(I) sulphide in air. Construct a symbol equation
for this extractive method. ___________________________________________
_______________________________________________________________________
[2]
2. Silver is highly resistant to corrosion by atmospheric oxygen.
However, silver slowly 'tarnishes' in the presence of either sulfur or
compounds of sulfur; the corrosion product is known as silver tarnish
(Ag2S). One method of removing this tarnish from a silver object is to
rub its surface with aluminium (whose oxide layer has been removed by
suspension in aqueous sodium chloride). Construct the symbol equation
for this unusual application of a displacement reaction. ______________
_______________________________________________________________________
[2]
3. Silver's attractive appearance, high resistance to corrosion, and
high electrical conductivity has resulted in its extensive use as an
electroplating metal. In silver-electroplating, the object to be plated
is made the cathode of an electrolysis cell which contains a silver
anode and an electrolyte of aqueous silver(I) nitrate.
Write an ionic equation for the reaction which occurs at the cathode.
_______________________________________________________________________
[1]
What energy change occurs in the endergonic process of electrolysis?
_______________________________________________________________________
[2]
4. Silver(I) halides are photosensitive (a property which is exploited
in black-and-white photography and in photochromic lenses); e.g., the
exposure of silver(I) bromide to light energy results in photolysis:

What energy change occurs in the endergonic process of photolysis?
_______________________________________________________________________
[2]
5. White photographic paper, containing silver(I) bromide, darkens on
exposure to light as black metallic silver particles are formed. A
chemist, who was researching new types of 'photo-paper', investigated
two related hypotheses: 'The speed (S) at which photographic paper "X"
darkens to standard grey colour decreases in linear proportion to the
distance (D) from a light source; i.e., S = k × D + c', and 'The speed
(S) at which photographic paper "X" darkens to standard grey colour
increases in linear proportion to the inverse square of the distance
(D-²) from a light source; i.e., S = k × D-² + c'; the Table shows a
summary of the chosen conditions and raw data.
Constants: photographic paper "X" (36 cm²); 30 W fluorescent light;
standard grey colour (Munsell 6); ambient temperature (17°C); distance
(D) measured from light bulb side-surface to paper.
Distance (D) / mm |
45 |
58 |
71 |
84 |
97 |
97 |
97 |
Distance-² (D-²) / m-² |
|
|
|
|
|
|
|
Time (t) / s |
144 |
185 |
214 |
236 |
232 |
254 |
249 |
Speed (S) / ms-¹ |
69 |
54 |
47 |
42 |
43 |
39 |
40 |
(a) Plot a graph, with distance (D) as the independent variable, and
then draw a best curve through as many points as is sensible.
[3]
Construct a precisely worded conclusion for distance (D) as the
independent variable. _________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
Distance (D) / mm
0 50 60 70 80 90 100
| | | | | | |
70_|/\/|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
S _|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
p 60_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
e _|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
e _|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
d _|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
(S) 50_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
/ _|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
ms-¹ _|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
40_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
\|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0 _/|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | |
0 100 200 300 400 500 600
Distance-² (D-²) / m-²
(b) Calculate the values for the inverse-square of the distance (D-²),
and insert these data in the Table.
[3]
Plot a (second) graph, with the inverse-square of distance (D-²) as the
independent variable, and then draw a best straight line through as
many points as is sensible.
[3]
Determine the gradient of this second graph; this value, 'k', is the
proportionality constant in the linearly proportional relationship
S = k × D-² + c. ______________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Construct a precisely worded conclusion for the inverse-square of
distance (D-²) as the independent variable. ___________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[5]
METALS: STRONTIUM
Strontium, a relatively rare element in the Earth's crust (0.04%),
occurs mainly as the sulfate or carbonate (e.g., in the ores celestite
and strontianite, respectively). Strontium compounds have neither any
large-scale industrial uses nor any known biological rôles. However,
partly because of the observed and predicted similarities between
calcium and strontium ions, and partly because calcium ions have been
shown to be essential to all living organisms, strontium ions could be
expected to affect diverse biochemical processes.
[.. K > Ba > Sr > Ca > Na > Mg > Al > Zn > Fe > Pb > (H) > Cu > Hg ..]
1. Based on strontium's position in the reactivity series, suggest how
the metal could be extracted from its chloride. _______________________
_______________________________________________________________________
[2]
2. This first Table shows the temperatures necessary to induce, and
the heat energy changes accompanying, the thermal decomposition of four
Group 2 carbonates.
|
BaCO3 |
SrCO3 |
CaCO3 |
MgCO3 |
Decomposition temperature / °C |
1360 |
1290 |
900 |
400 |
Heat energy change (DH) / kJ mol-¹ |
269 |
235 |
178 |
101 |
A radiochemist used the carbon-14 isotope to prepare a small amount of
radioactive labelled strontium carbonate, Sr14CO3. Using the apparatus
shown, a sample of this labelled compound was heated; the evolved gas
passed into limewater, and the precipitate collected by filtration.
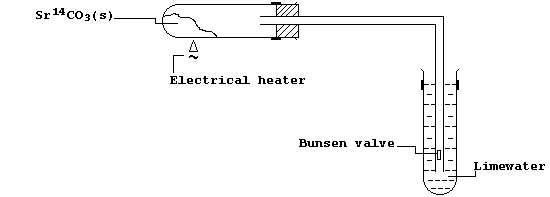
(a) Ensuring you indicate the radioactive-labelled carbon, complete and
label the energy level diagram for the endothermic decomposition of
strontium carbonate.
Energy ___
___________________
___________________
____________ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
____________
______________________________________________
Path of reaction
[5]
(b) Construct the symbol equation for the reaction of the evolved gas
with limewater. _______________________________________________________
_______________________________________________________________________
[2]
3. Strontium carbonate could be reasonably viewed as an 'analogue' of
calcium carbonate, which is a major component of the exoskeletons of
various protoctistans, sponges, corals, crustaceans, and molluscs.
Temporary hard water results from the slow reaction of rainwater with
naturally occurring calcium carbonate; its 'strontium equivalent' is: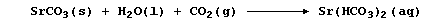
Complete this second Table to show further strontium analogues.
|
Calcium compound |
Strontium analogue |
Mammalian endoskeleton |
Ca5(PO4)3OH(s) |
Sr5(PO4)3OH(s) |
Crustacean exoskeleton |
CaCO3(s) |
SrCO3(s) |
Permanent hard water |
|
|
Temporary hard water |
|
|
'Scale' or 'fur' |
|
|
Limewater |
|
|
[2]
Construct the symbol equation for each of these strontium equivalents.
Softening permanent hard water ________________________________________
_______________________________________________________________________
Softening temporary hard water ________________________________________
_______________________________________________________________________
Removing scale with aqueous methanoic acid, HCOOH(aq) _________________
_______________________________________________________________________
[6]
4. Aqueous strontium hydroxide contains the ions Sr2+(aq), OH1-(aq),
and H1+(aq). When this solution is electrolyzed, using carbon-graphite
electrodes, these reactions occur: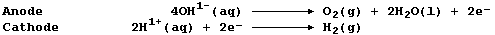
Explain, using a symbol equation, each of the following observations
made during such an electrolysis experiment.
The mass of the anode decreased. ______________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
The solution turned milky during the initial stages of electrolysis. __
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
The milky suspension turned clear if electrolysis was continued. ______
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
5. One radioactive isotope of strontium, Sr-90, has a half-life of 28
years and is a b-emitter (i.e., it decays spontaneously, emitting
radiation in the form of high speed electrons). This isotope, one of
the most dangerous waste products of the nuclear industry, is a fission
product in nuclear explosions and in reactors of nuclear power plants.
Suggest what might happen to any compounds of strontium-90 ingested by
a mammal. _____________________________________________________________
_______________________________________________________________________
[2]
If 0.16 g of strontium-90 was absorbed into the exoskeleton of a coral
(e.g., Corallum rubrum), how much would remain after 84 years? ________
_______________________________________________________________________
[2]
Name one process in autotrophs or saprotrophs that might be affected by
strontium-90. _________________________________________________________
[1]
METALS: CADMIUM
Cadmium, a very rare element in the Earth's crust (0.00002%), occurs
mainly as the sulfide or carbonate in zinc ores. Compounds of cadmium,
in contrast to those of zinc, appear to have little or no biological
rôle; so, inevitably, cadmium(II) ions accumulate up the trophic levels
because very few living organisms have evolved methods for egesting or
excreting these ions. In addition, partly because of the observed and
predicted similarities between zinc and cadmium ions, and partly
because enzymes containing zinc(II) ions are ubiquitous, cadmium ions
could be expected to affect diverse biochemical processes.
[.. K > Ca > Na > Al > Zn > Cd > Fe > Ni > Pb > (H) > Cu > Hg > Ag ..]
1. The extraction of cadmium from zinc ores is fairly complex, but the
final process involves chemical reduction of aqueous cadmium sulfate
with zinc (DE = -70 kJ mol-¹). For this redox reaction, construct the:
symbol equation; redox half equations; and net ionic equation. ________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
Use the net ionic equation to complete and label this energy level
diagram.
Energy
____
__________________ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
__________________
__________________
__________________
__________________________________________________
Path of reaction
[4]
2. Currently, the major use of cadmium is in rechargeable 'nicad'
batteries. Explain the direction of electron flow when a Ni-Cd cell is
in use (i.e., transducing chemical to electrical energy). _____________
_______________________________________________________________________
[2]
3. A research chemist investigated the following hypothesis: 'The
speed (S) of the displacement reaction between cadmium and aqueous
sulfuric acid increases in linear proportion to the temperature (T) of
the acid; i.e., S = k × T + c'; the Table shows a summary of the chosen
conditions and raw data (no duplicate experiments were executed).
Constants: amount (0.050 mol) of cadmium granules (spheres with surface
areas of 4.2 ± 0.2 mm³); concentration (1.00 mol dm-³) and volume
(75 cm³) of aqueous sulfuric acid; absence of catalysts; thermostatted
water-bath; room temperature (295 K) and pressure (100 kPa); volume of
dihydrogen collected via a gas syringe (50 cm³).
Temperature (T) / K |
295 |
307 |
319 |
331 |
343 |
Reaction time (t) / s |
77 |
40 |
25 |
21 |
17 |
Reaction speed (S) / ms-¹ |
13 |
|
|
|
|
(a) State one reason why higher values of the independent variable were
not examined. _________________________________________________________
[1]
(b) Calculate the missing values for the dependent variable, and insert
these data into the above Table.
[2]
(c) Label both axes, with the independent variable on the horizontal
axis (cela va sans dire?), and then plot the data points.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
60_|_|_|_ Aqueous |_|_|_|_|_| Aqueous |_|_|_|_|_|_|_|_|_|_|_|
|_| sulfuric acid |_|_|_sulfuric acid |_|_|_|_|_|_|_|_|_|
_|_(1.00 mol dm-³)_|_|_|(1.00 mol dm-³) _|_|_|_|_|_|_|_|_|_|
|_|_|_| + |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
50_|_|_| Cu(s) |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_(0.001 mol) |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
40_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
30_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
20_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
10_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| Aqueous |_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| ethanoic acid |
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| (2.00 mol dm-³) |
0_|/\/\/|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | |
0 293 303 313 323 333 343
[4]
Draw a best straight line through as many points as is sensible, and
then determine the gradient of the (plotted) graph; this value, 'k', is
the proportionality constant in the linearly proportional relationship
S = k × T + c. ________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Write a precisely worded conclusion based on the (plotted) graph. _____
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[5]
(d) Sketch the graph that would be obtained if one of the constants was
changed separately, and explain why each of these graphs differs from
that obtained for the set of constants shown with the Table.
Aqueous sulfuric acid (1.00 mol dm-³) and copper (0.001 mol) __________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
Aqueous ethanoic acid (2.00 mol dm-³) _________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
METALS: MOLYBDENUM
Molybdenum, a rare element in the Earth's crust (0.002%), is usually
found as a sulfide (e.g., in the ore molybdenite). This transition
metal, which has a very high melting point (2617°C) and a high density
(10.28 g cm-³), forms coloured compounds in several oxidation states
[e.g., Mo(II), Mo(III), and Mo(VI)]. Molybdenum has been shown to be
essential to all living organisms, but its particular importance in
biological chemistry centres on nitrogenase; this molybdenum-containing
enzyme, which is present in nitrogen-fixing bacteria (e.g., Rhizobium),
is involved in the reduction of atmospheric nitrogen to ammonia.
[.. K > Ca > Mg > Al > Zn > Fe > Mo > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. The extraction of molybdenum from its ores is very complex, and so
only a simplified summary is presented here:
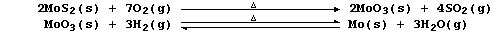
(a) Explain what the double-headed arrow means in the second equation.
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) Suggest a different reductive method of obtaining molybdenum from
molybdenum(VI) oxide. _________________________________________________
[1]
2. Many leguminous plants have evolved symbiotic relationships with
nitrogen-fixing bacteria living in their root nodules; a legume uses
the ammonium ions absorbed from these bacteria to biosynthesize amino
acids, ATP, chlorophylls, and nucleic acids.
(a) Name two legumes. _________________________________________________
(b) Suggest in what form chemical energy is absorbed by these bacteria
from the legume. ______________________________________________________
(c) Cereal farmers will clearly reduce their dependence on artificial
nitrogenous fertilizers if they introduce legumes as 'break crops'.
Explain one disadvantage inherent in the use of such fertilizers. _____
_______________________________________________________________________
_______________________________________________________________________
[5]
3. Industrial nitrogen-fixation is commonly achieved by the Haber
process, which is usually summarized by the following equation: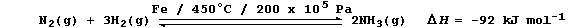
Explain one reason why each of the following conditions speed up the
above reaction - so saving time (and, therefore, money).
Powdered iron _________________________________________________________
_______________________________________________________________________
High temperature ______________________________________________________
_______________________________________________________________________
High pressure _________________________________________________________
_______________________________________________________________________
[6]
4. Molybdenum(VI) oxide is used as a co-catalyst in the industrial
oxidation of a propene and ammonia mixture to propenenitrile.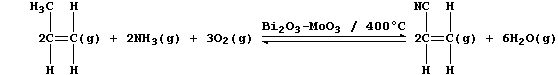
Propenenitrile, better known as acrylonitrile, is the monomer used to
manufacture which polymer? ____________________________________________
[1]
5. Molybdenum's position in the reactivity series can be determined
by measuring potential differences of electrochemical cells; the Table
shows some typical data, together with the electrical conductivities of
the selected metals at ambient temperatures.
Metal(M) |
Al |
Ti |
Cr |
Ga |
In |
Mo |
Rh |
Ir |
Au |
Cell p.d.(Au-M) /V* |
3.16 |
2.71 |
2.24 |
2.06 |
1.84 |
1.70 |
0.74 |
0.34 |
0.00 |
Cond. / MW cm-¹ |
40.0 |
2.6 |
7.9 |
7.4 |
12.5 |
20.0 |
23.2 |
21.2 |
48.8 |
* The data, which have been obtained under standard conditions (25°C;
pH = 0), refer to the oxidation half-reaction M(s) ——® M3+(aq) + 3e- |
(a) Noting that each quantitative dependent variable is a linear scale
on the vertical axis, and that the qualitative independent variable is
shown as separate blocks on the horizontal axis, complete this double
bar-graph of these data.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
|_| |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| |/|
|_| |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| |/|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
C 3.0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_60 E
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| l
l |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| e
l |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| c
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| t
p 2.5_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_50 r
o |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| i
t |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| c
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| a
n |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| l
t 2.0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_40
i |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| c
a |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| o
l |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| n
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| d
d 1.5_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_30 u
i |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| c
f |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| t
f |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| i
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| v
r 1.0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_20 i
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| t
n |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| y
c |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
e |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| /
0.5_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_10
(Au-M) |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_| MW cm-¹
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
/ |_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
V 0.0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_0
Al Ti
Metal (M)
[7]
(b) Molybdenum may prove attractive as a component of commercial cells,
particularly as it is at least ten times more abundant in the Earth's
crust than cadmium, mercury, or silver (which are used in Ni-Cd, Hg-Zn,
and Ag-Zn cells, respectively). For a specialized application which
required a p.d. of about 1 V, molybdenum could be paired with either of
which two metals? ____________________________________________________
[2]
METALS: VANADIUM
Vanadium, a relatively rare element in the Earth's crust (0.01%), is
usually found as a sulfide (e.g., in the ore patronite). This
transition metal, which has a high melting point (1890°C) and a high
density (6.09 g cm-³), forms coloured compounds in several oxidation
states [e.g., V(II), V(III), V(IV), and V(V)]. Although the metal is
used to manufacture relatively small quantities of 'vanadium' steels
that have high tensile strengths, the element's particular importance
in industrial chemistry centres on the use of vanadium(V) oxide as a
catalyst for the oxidation of sulfur dioxide to sulfur trioxide (a
key step in the manufacture of sulfuric acid).
[.. Ca > Na > Mg > Al > V > Zn > Fe > Sn > Pb > (H) > Cu > Hg > Ag ..]
1. The extraction of vanadium from its various ores is very complex,
but the final process involves chemical reduction of vanadium(V) oxide
with molten calcium. Construct the symbol equation for this reaction.
_______________________________________________________________________
[2]
Calcium is preferred to carbon as the reducing agent, for two reasons:
one, a lower furnace temperature can be used because the redox reaction
is more exothermic; and two, carbon forms compounds with vanadium at
high temperatures. Suggest one disadvantage in the use of calcium as a
reducing agent. _______________________________________________________
[1]
2. Shown below is a diagram of four electrolytic cells connected in
series; nominally, each cell contained the vanadium ions as stated.
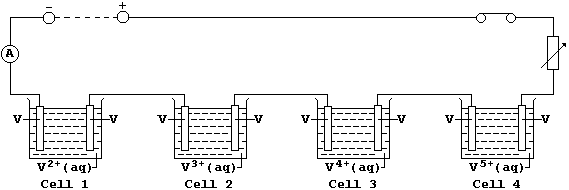
[Q = n × z × F and Q = I × t, where: Q, measured in coulombs (C), is
the quantity of electricity; n is the number of moles of substance
evolved at the electrode; z is the charge on the ion; F is a constant,
with a value of 96500 C mol-¹; I, measured in amps (A), is the current;
and t, measured in seconds (s), is the time.]
(a) In 60 minutes, 1.275 g of vanadium were deposited at the cathode of
cell 1. Determine the current (I) that flowed in this circuit - as
follows.
Calculate the number of moles (n) of vanadium deposited at the cathode
of cell 1. ____________________________________________________________
Calculate the quantity of electricity (Q) required to deposit this
number of moles. ______________________________________________________
And finally, calculate the current (I) that flowed in the circuit. ____
_______________________________________________________________________
[6]
(b) Complete the Table below, calculating the values where appropriate.
|
Cell 1 |
Cell 2 |
Cell 3 |
Cell 4 |
Charge on vanadium ion |
2+ |
3+ |
4+ |
5+ |
Vanadium deposited (n) / mol |
|
|
|
|
[3]
3. The radioactive isotope vanadium-49 has a half-life of 330 days. A
sample tube, sealed in a lead-lined chamber for 1320 days, was found to
contain 0.125 g of this isotope.
(a) Calculate the mass of the isotope that was present originally. ____
_______________________________________________________________________
_______________________________________________________________________
[3]
(b) Using the data calculated above, plot a graph of mass of isotope
(vertical axis) against the number of half-lives elapsed, and then draw
the best curve through the points.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | |
[6]
4. Sulfur trioxide is manufactured by the Contact process, which is
usually summarized by the following equation: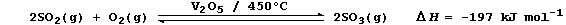
(a) Explain the effects, on the rate of this reaction and the yield of
sulfur trioxide, of using:
The catalyst __________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
A high pressure _______________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
A higher temperature __________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
(b) A research chemist prepared a sample of labelled sulfur trioxide,
using the isotope oxygen-18. This sample was heated with a catalytic
quantity of vanadium(V) oxide, at 450°C in a closed system, until the
composition of the mixture remained constant. For the reaction studied,
construct the symbol equation complete with heat energy change. _______
_______________________________________________________________________
_______________________________________________________________________
[3]
METALS: TITANIUM
Titanium, the seventh most abundant metal in the Earth's crust (0.6%),
is usually found as an oxide (e.g., in the ores rutile and ilmenite).
This transition element, which has a high melting point (1660°C) but
only a moderately-high density (4.51 g cm-³), shows variable oxidation
states [e.g., (coloured) Ti(III) and (colourless) Ti(IV)].
[.. K > Ca > Na > Mg > Al > Ti > Zn > Fe > Pb > (H) > Cu > Hg > Ag ..]
1. The extraction of titanium from its principal ores is complex, but
the final process involves chemical reduction of titanium(IV) chloride
with magnesium at 1050°C in an atmosphere of argon.
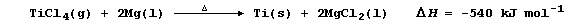
(a) Titanium metal is expensive, because its extraction involves high
costs in terms of energy, raw materials, and ensuring safety. From a
consideration of the above equation, suggest and explain one possible
way of reducing energy costs. _________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
Suggest one reason why it is necessary to carry out this reduction in
an atmosphere of argon. _______________________________________________
_______________________________________________________________________
[1]
(b) The covalent compound titanium(IV) chloride, a liquid at room
temperature, rapidly hydrolyzes to give an acidic solution and a white
solid (Mr = 80). Construct the symbol equation for this hydrolysis. ___
_______________________________________________________________________
[2]
2. Titanium, despite its high position in the reactivity series, does
not react with water, steam, or cold dilute acids; this observed lack
of reaction is due to the presence of a protective oxide layer. Explain
why this oxide layer reduces titanium's rate of reaction with several
reactants to effectively zero. ________________________________________
_______________________________________________________________________
[2]
3. There is a demand for alloys which incorporate both titanium and
aluminium. Suggest two properties of such alloys. _____________________
_______________________________________________________________________
[2]
4. During the past few decades, manufacturers have used alternatives
to lead compounds as pigments in paint; in particular, there is now
extensive use of titanium(IV) oxide - which is very stable, non-toxic,
and brilliant white. Perhaps surprisingly, this titanium pigment is not
usually obtained from purified rutile (TiO2), but from ilmenite.
(a) Analysis showed that a 5.00 g sample of pure ilmenite had this
composition by mass: iron, 1.86 g; titanium, 1.56 g; and oxygen as the
only other element present.
|
Fe |
Ti |
O |
Mass combining (m) / g |
|
|
|
Molar mass (M) / g mol-¹ |
|
|
|
Number of moles combining (m ÷ M)
|
|
|
|
Simplest ratio of number of moles |
|
|
|
Complete the above Table, so as to determine the empirical formula of
ilmenite. _____________________________________________________________
[5]
(b) Suggest one reason why there has been a decrease in the use of lead
compounds in the manufacture of paints (and petrol). __________________
_______________________________________________________________________
[1]
5. A characteristic reaction of alkenes is addition polymerization.
One of the many versatile reaction schemes used to synthesize addition
polymers is summarized below: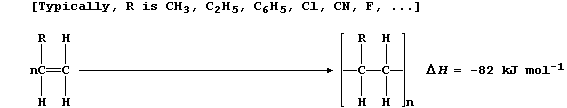
The general formula given above for an addition polymer is, however,
a shade misleading because analyses have shown that most addition
polymers can be obtained in at least three different forms: i.e.,
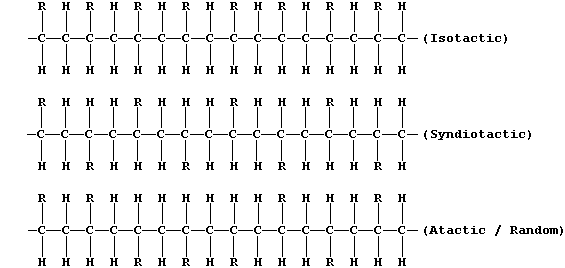
(a) What name is given to compounds which have the same molecular
formula but different structural formulae? ____________________________
[1]
(b) Suggest two physical methods which could be used to distinguish
between the different forms of an addition polymer (containing the same
functional group R). __________________________________________________
[2]
(c) Several transition metal compounds are known to act as catalysts
for the polymerization of alkenes; their use usually results in higher
yields of the isotactic and syndiotactic forms of addition polymers.
Titanium(IV) chloride is often stated to be a polymerization catalyst:
however, experiments have shown that the true catalyst is titanium(III)
chloride [which is formed by adding triethylaluminium, (C2H5)3Al, to a
reaction mixture of titanium(IV) chloride and alkene]. State the two
measurements that are required to determine whether a substance is
acting as a catalyst (apart from a comparison of reaction rates with
and without the substance). ___________________________________________
_______________________________________________________________________
[2]
6. Naturally occurring titanium contains five isotopes; the three most
abundant are Ti-46 (8.0%), Ti-47 (7.3%), and Ti-48 (73.8%). The most
stable radioactive isotope, titanium-44, has a half-life of 47 years.
Calculate the exact relative atomic mass of a sample of titanium which
contains only the three most abundant isotopes. _______________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
METALS: NICKEL
Nickel, which is relatively rare in the Earth's crust (0.008%), is
usually found as a sulfide (e.g., in the ore millerite). This element
is a typical transition metal, as evinced by its high melting point
(1535°C), high density (8.91 g cm-³), variable oxidation states [e.g.,
Ni(II) and Ni(III)], formation of coloured compounds (which are often
green), and catalytic activity (e.g., it is used in the hydrogenation
and dehydrogenation of organic compounds).
[.. K > Cs > Ca > Na > Mg > Al > Fe > Ni > Sn > (H) > Cu > Hg > Ag ..]
1. Suggest how nickel can be extracted from nickel(II) oxide, which is
obtained by roasting its sulfides in air. _____________________________
_______________________________________________________________________
[1]
2. Perhaps coincidentally, the thermal stabilities of nitrates appear
to parallel the reactivity series. For example, mercury(II) nitrate
decomposes on very gentle heating, to give a silvery liquid, nitrogen
dioxide, and dioxygen: whereas, nickel(II) nitrate decomposes only on
moderately strong heating, to give a black solid, nitrogen dioxide, and
dioxygen: and, typical of Group 1 nitrates, caesium nitrate decomposes
only on very strong heating, to give a pale-yellow solid and dioxygen.
(a) Construct the symbol equation for each of these decompositions.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
(b) The volume (V1) of one mole of any gas at room temperature (25°C =
298 K; T1) and pressure (100 kPa; P1) is 24000 cm³; furthermore, the
following relationship holds true for gases:
P1 × V1 P2 × V2
¾¾¾¾¾ = ¾¾¾¾¾
T1 T2
Determine the volume (V2) of nitrogen dioxide, at room temperature and
low pressure (5 kPa; P2), obtained from the thermal decomposition of
3.66 g of nickel(II) nitrate - as follows.
Calculate the molar mass of nickel(II) nitrate. _______________________
_______________________________________________________________________
Calculate the number of moles of nickel(II) nitrate in 3.66 g of the
compound. _____________________________________________________________
Using the symbol equation, determine the number of moles of nitrogen
dioxide obtained from this number of moles of nickel(II) nitrate. _____
_______________________________________________________________________
Calculate the volume (V1) of gas obtained at room temperature (T1) and
pressure (P1). ________________________________________________________
And finally, using the above relationship, calculate the volume (V2) of
gas at the decreased pressure (P2). ___________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[9]
3. When powdered nickel is added to aqueous mercury(II) nitrate, the
grey solid rapidly dissolves, the colourless solution changes to green,
a silvery liquid forms, and the temperature of the solution increases.
Construct the net ionic equation for this redox reaction, complete with
a qualitative indication of the heat energy change. ___________________
_______________________________________________________________________
[3]
4. Suggest one reason why aqueous solutions of metal nitrates should
not be discharged into the environment. _______________________________
_______________________________________________________________________
[1]
5. Shown below is a diagram of an electrolytic cell used to nickel-
plate an aluminium object.
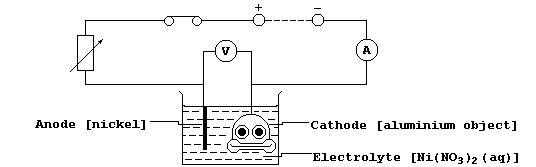
[Q = n × z × F and Q = I × t, where: Q, measured in coulombs (C), is
the quantity of electricity; n is the number of moles of substance
evolved at the electrode; z is the charge on the ion; F is a constant,
with a value of 96500 C mol-¹; I, measured in amps (A), is the current;
and t, measured in seconds (s), is the time.]
(a) The mass (m) of the object increased by 0.383 g in 20 minutes.
Write an ionic equation for the reaction which occurs at the cathode.
_______________________________________________________________________
Calculate the number of moles (n) of nickel deposited at the cathode.
_______________________________________________________________________
Calculate the quantity of electricity (Q) required to deposit this
number of moles. ______________________________________________________
And finally, calculate the current (I) that flowed in the circuit. ____
_______________________________________________________________________
[7]
(b) State and explain what would be observed for each of the following,
if the polarities of the above circuit were reversed.
Nickel strip __________________________________________________________
_______________________________________________________________________
Aluminium object ______________________________________________________
_______________________________________________________________________
Electrolyte ___________________________________________________________
_______________________________________________________________________
[6]
6. Nickel's use as a catalyst is exemplified by the dehydrogenation of
ethylbenzene to phenylethene. [This alkene, better known as styrene, is
the monomer in the manufacture of poly(phenylethene).]
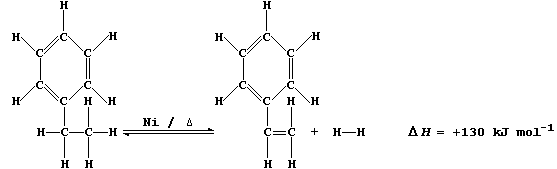
Suggest and explain two advantages in using a high temperature in the
above reaction. _______________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
METALS: MANGANESE
Manganese, the eighth most abundant metal in the Earth's crust (0.09%),
occurs mainly in the ore pyrolusite (impure MnO2). This transition
element, which has a high melting point (1244°C) and a high density
(7.44 g cm-³), forms compounds in several oxidation states [e.g.,
(pale-pink) manganese(II) sulfate, (red) manganese(III) sulfate,
(brown) manganese(IV) oxide, and (purple) potassium manganate(VII)].
[.. K > Ca > Na > Mg > Al > Mn > Zn > Fe > Sn > (H) > Cu > Hg > Ag ..]
1. Manganese is produced in two forms: ferromanganese, a carbon-
containing alloy used to manufacture hard 'manganese' steels; and the
pure metal, which is used to manufacture non-ferrous alloys (e.g.,
Duralumin and Manganin).
(a) Ferromanganese is formed when a mixture of haematite, pyrolusite,
coke, and limestone is heated in a furnace; the proportion of iron to
manganese in the alloy is dependent on the purity and ratio of the ores
in the mixture. The following symbol equation summarizes the conditions
used to obtain this alloy with a manganese content of 80%:
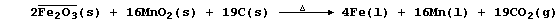
Suggest the formulae of two other substances formed in the furnace. ___
_______________________________________________________________________
[2]
(b) The pure metal, for which there is very little demand, used to be
obtained exclusively by the chemical reduction of purified pyrolusite: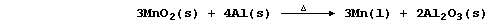
Construct the net ionic equation for the above redox reaction. ________
_______________________________________________________________________
Suggest one reason why aluminium is preferred to carbon as the reducing
agent. ________________________________________________________________
[3]
Partly because of the viciously exothermic nature of the above reaction
(DH = -1791 kJ mol-¹), the currently preferred method of obtaining the
pure metal is by the electrolytic reduction of aqueous manganese(II)
sulfate; this salt is obtained by the oxidation of concentrated
sulfuric acid with manganese(IV) oxide: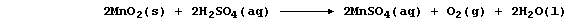
Explain, using a redox half equation, why the above equation summarizes
a redox reaction (and not a neutralization). __________________________
_______________________________________________________________________
[2]
2. Manganese(IV) oxide and potassium manganate(VII) are both used
extensively as oxidizing agents in laboratories and in industry.
(a) Although too expensive to be used on an industrial scale, the
standard laboratory preparation of chlorine gas involves the oxidation
of concentrated hydrochloric acid with manganese(IV) oxide. Construct
the symbol equation for this redox reaction. __________________________
_______________________________________________________________________
[2]
(b) Several companies use potassium manganate(VII) to purify water for
domestic use; aqueous manganate ions, MnO41-(aq), which can penetrate
cell walls and membranes, act as biocides by oxidizing a range of
essential biological molecules. This oxidizing agent is often preferred
to chlorine, for two reasons: one, it does not affect the taste of the
water; and two, the manganese(IV) oxide produced acts as a coagulating
agent for suspended particles. Write a redox half equation to represent
the change in oxidation state of manganese. ___________________________
_______________________________________________________________________
[1]
Name one other coagulating agent commonly used in water purification.
_______________________________________________________________________
[1]
3. Aqueous hydrogen peroxide decomposes slowly at ambient temperature;
the equation for this decomposition can be written as follows: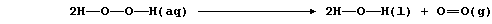
(a) Complete this Table using these bond energies (in kJ mol-¹):
H-O (463); O-O (146); and O=O (497).
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
|
|
|
|
|
|
|
|
Total = |
Total = |
[3]
Calculate the heat energy change (DH) for the above decomposition. ____
_______________________________________________________________________
[2]
(b) Catalase, an iron-containing enzyme with a structure very similar
to haemoglobin, and manganese(IV) oxide both speed up the decomposition
of hydrogen peroxide; though the enzyme is considerably more effective
than the inorganic catalyst, as might be expected. Complete and label
this energy level diagram for the decomposition of hydrogen peroxide
using these two different catalysts.
Energy ____
____
____
__________ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
__________
__________________
__________________
__________________________________________________
Path of reaction
[6]
4. Photosynthesis in plants can be summarized by this equation: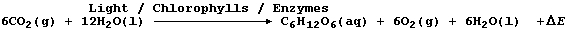
In one of the light-dependent reactions, a manganese-containing enzyme
is involved in the oxidation of water to form the oxygen gas;
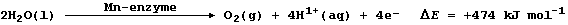
(a) Experiments have shown that water, and not carbon dioxide, is the
source of oxygen atoms in the oxygen gas formed. Using symbols, suggest
what 'type' of water was used in these experiments. ___________________
(b) Suggest the meaning of the term '+ DE'. ___________________________
_______________________________________________________________________
[2]
5. In mammals, urea is formed by the deamination of excess amino
acids; a manganese-containing enzyme, known as arginase, is involved in
this biochemical process.
(a) Name the other excretion product formed by the deamination of
excess amino acids. ___________________________________________________
(b) Where in a mammal is urea biosynthesized? _________________________
[2]
METALS: CHROMIUM
Chromium, a relatively rare element in the Earth's crust (0.01%),
occurs mainly in the ore chromite (impure FeCr2O4). This transition
metal, which has a high melting point (1857°C) and a high density
(8.89 g cm-³), forms compounds in several oxidation states [e.g., (red)
chromium(II) ethanoate, (green) chromium(III) sulfate, (brown-black)
chromium(IV) oxide, and (orange) potassium dichromate(VI)].
[.. K > Ca > Na > Mg > Al > Zn > Cr > Fe > Pb > (H) > Cu > Hg > Ag ..]
1. Chromium is produced in two forms: ferrochrome, a carbon-containing
alloy used to manufacture stainless and hard 'chromium' steels; and the
pure metal, which is used to electroplate steel and to manufacture
non-ferrous alloys (e.g., Nichrome).
(a) Ferrochrome is formed when a mixture of chromite, coke, and
limestone is heated in a furnace. Complete the symbol equation below,
so as to summarize the conditions used to obtain this alloy:
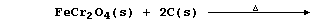
[1]
(b) The extraction of chromium from chromite is complex, but the final
process involves chemical reduction of (green) chromium(III) oxide with
aluminium. Construct the symbol equation for this redox reaction. _____
_______________________________________________________________________
[2]
The metal is electrolytically purified using impure chromium as the
anode, pure chromium as the cathode, and an aqueous chromium salt as
the electrolyte. State the energy change which occurs at the cathode.
_______________________________________________________________________
[2]
(c) Shown below is a diagram of an electrolytic cell used to chromium-
plate a toy-car bumper.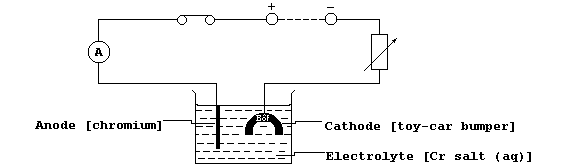
[Q = n × z × F and Q = I × t, where: Q, measured in coulombs (C), is
the quantity of electricity; n is the number of moles of substance
evolved at the electrode; z is the charge on the ion; F is a constant,
with a value of 96500 C mol-¹; I, measured in amps (A), is the current;
and t, measured in seconds (s), is the time.]
The mass (m) of the toy-car bumper increased by 0.0793 g when a direct
current of 1.0 A flowed in the above circuit for 900 s. Calculate the:
Number of moles (n) of chromium deposited at the cathode. _____________
_______________________________________________________________________
Quantity of electricity (Q) which flowed through this circuit. ________
_______________________________________________________________________
Charge (z) on the chromium ion of the electrolyte. ____________________
_______________________________________________________________________
[6]
(d) To prevent rusting, iron objects are sometimes plated with chromium
rather than galvanized with zinc. Suggest one reason why chromium is
used, despite zinc being cheaper and more effective as a sacrificial
anode. ________________________________________________________________
[1]
2. Naturally occurring chromium contains four isotopes: Cr-49 (2.3%),
Cr-50 (4.3%), Cr-52 (83.8%), and Cr-53 (9.6%). Calculate the exact
relative atomic mass of chromium. _____________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
3. Suggest what observations should be made, and construct the symbol
equation, for the reaction of powdered chromium with each of these
reactants [consider chromium(III) to be the preferred oxidation state].
Steam _________________________________________________________________
_______________________________________________________________________
[4]
Aqueous copper(II) sulfate ____________________________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
4. Chromium(III) oxide, an amphoteric substance, is used as a green
pigment in paints and as a catalyst for the polymerization of alkenes.
The pure oxide can be obtained by the thermal decomposition of several
chromium salts; e.g., as can be vividly demonstrated in the 'volcano'
experiment, ammonium dichromate(VI) thermally decomposes as follows:
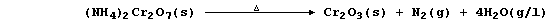
(a) State briefly the meaning of the term 'amphoteric oxide'. _________
_______________________________________________________________________
[1]
(b) Fairly strong heating of anhydrous chromium(III) nitrate results in
the formation of a green solid, a brown gas which turns damp pH paper
red, and a colourless gas which relights a glowing spill. Construct the
symbol equation for the thermal decomposition of chromium(III) nitrate.
_______________________________________________________________________
[2]
5. Dichromates are used extensively as oxidizing agents in industry
and in laboratories; e.g., acidified sodium dichromate(VI) oxidizes
ethanol to ethanoic acid. Complete this scheme to show the structural
formula of the carboxylic acid obtained by oxidizing hexan-1-ol.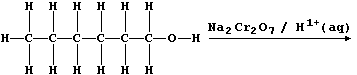
[2]
Hexanoic acid, together with C7, C8, C9, C10, and C12 straight-chain
carboxylic acids, is a component of the trail pheromone of at least one
species of ant (Lasius fuliginosus). Speculate on the purpose of the
'trail'. ______________________________________________________________
[1]
6. Chromium ions are potential pollutants, because the amounts used
industrially are large compared to the ambient concentrations tolerated
by living organisms. These ions are certainly toxic and may also be
carcinogenic: nevertheless, in trace quantities, Cr(III) ions have been
shown to be essential in mammals; e.g., together with the hormones
insulin and glucagon, they help maintain the homeostatic concentration
of glucose in the blood.
(a) In which organ is insulin biosynthesized? _________________________
(b) In which organ does insulin stimulate the conversion of soluble
glucose to insoluble glycogen? ________________________________________
(c) Name one (non-infectious) disease caused by a deficiency of insulin
or chromium(III) ions. ________________________________________________
[3]
METALS: PLATINUM
Platinum, one of the rarest elements in the Earth's crust (0.0000005%),
is usually found either native or as an alloy in various ores. This
element is a typical transition metal, as evinced by its high melting
point (1772°C), high density (21.45 g cm-³), variable oxidation states
[e.g., Pt(II) and Pt(IV)], and catalytic activity (e.g., Pt-Al2O3 is
used as a co-catalyst in the cracking of petroleum oil fractions).
[.... Zn > Fe > Co > Ni > (H) > Cu > Hg > Ag > Rh > Pd > Ir > Pt > Au]
1. There is no routine method for the extraction of platinum; the
processes used are highly dependent on the composition and quality of
the ores, which are invariably 'low-grade'. Currently, about 100 tonnes
of platinum are extracted each year. Calculate the mass of ore required
to obtain this mass of platinum, assuming that the platinum content of
the highest-grade ore is 0.000001%. ___________________________________
[1]
2. The central position of controlled experiments in science may give
the understandable impression that progress is achieved exclusively by
hypothesis testing (se non è vero, è ben trovato): but this is not the
case, either historically or contemporaneously. Thus, the discoveries
of the purple dye mauveine (1856), the antibiotic penicillin (1928),
the bactericide sulfonamide (1934), the sedative diazepam (1957), and
the carbon-allotrope buckminsterfullerene (1985) represent just five of
countless examples of serendipity; another is cisplatin, a platinum(II)
compound which unexpectedly showed anti-cancer activity in culture
cells (1968).
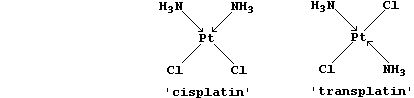
(a) Why are cisplatin and transplatin considered to be isomeric? ______
_______________________________________________________________________
[1]
(b) Following the serendipitous observation with cisplatin, scientists
synthesized and tested several thousand platinum(II) compounds in
attempts to obtain an anti-cancer drug with similar activity to, but
lower toxicity than, cisplatin (the so-called 'lead' compound). Draw
the structural formulae of two platinum(II) compounds which might show
similar chemical or biological properties to cisplatin. _______________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) Cancer, which is the uncontrolled mitotic division of cells, can
result from mutations (i.e., changes in the sequence of base pairs of
DNA). Accordingly, more recent studies have focused on the reactions
between platinum(II) compounds and the four nucleotide bases present in
DNA. Name these bases (usually represented by their initial letters:
'A', 'C', 'G', and 'T'). ______________________________________________
[4]
Mutations which occur in somatic cells may lead to cancer, but the
resulting genetic changes are not inheritable. By contrast, mutations
can be passed on to the offspring if they occur in the reproductive
cells of which two mammalian organs? __________________________________
[2]
3. Platinum's attractive appearance, high resistance to oxidation, and
high electrical conductivity has resulted in its use in jewellery and
as an electroplating metal. More importantly, despite (or because of)
its low chemical reactivity, platinum is used as a catalyst in at least
30 commercial processes. Indeed, the major current use of platinum is
as a co-catalyst in the 'catalytic convertors' of vehicle exhausts; one
of the many chemical reactions which occur in such convertors is:
Pt-Rh / D
2CºO(g) + 2NºO(g) —————————® O=C=O(g) + NºN(g)
(a) Complete this Table using these bond energies (in kJ mol-¹):
CºO (1069); NºO (627); C=O (745); and NºN (946).
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
|
|
|
|
|
|
|
|
Total = |
Total = |
[3]
Calculate the heat energy change (DH) for the above redox reaction. ___
_______________________________________________________________________
[2]
(b) Catalytic convertors undoubtedly reduce the emission of harmful
pollutants: but such benefits to the environment must be offset against
the potential destruction of ecosystems caused by extracting mineral
resources - particularly those of low natural abundance. Admittedly,
the precious metals are usually recycled when vehicles are scrapped or
convertors are rendered ineffective by 'catalyst poisoning': but, in
view of the increasing industrialization of the developing countries,
there must be doubts at to whether the use of precious metal catalysts
can be sustained. Consider, however, Vorsprung durch Biotechnik; the
lead compounds could be thermally robust enzymes isolated from species
of Archaebacteria (e.g., Methanococcus jannaschii), and recombinant DNA
technology could be used to alter the genes coding for these catalysts.
Suggest the meaning of the phrase 'thermally robust enzyme'. __________
_______________________________________________________________________
[1]
4. Finally, un divertissement ... The first organometallic compound
isolated was Zeise's salt (1825). Shown below is the structural formula
of this platinum(II)-ethene compound; the [PtCl3(C2H4)]1- ion is square
planar, with chloride at three corners and ethene at the other corner -
however, the ethene molecule is perpendicular to the PtCl3.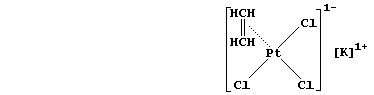
Zeise's salt has no commercial use, but related palladium(II)-alkene
compounds are catalysts in various industrial processes. Draw the
structural formula of [PdCl3(C2H4)]1-Na1+ and of trans-PdCl2(C2H4)2.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[3]
_______________________________________________________________________
_______________________________________________________________________
Comedy-Dramas for Year 10-12 Students in British Orthography :
[No. 1] [No. 2] [No. 3] [No. 4] [No. 5] [No. 6] [No. 7] [No. 8] [No. 9]
[Dr. Roger Peters : rpeters@wissensdrang.com ; Aufbau1 ; Home Page]