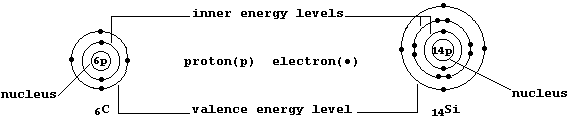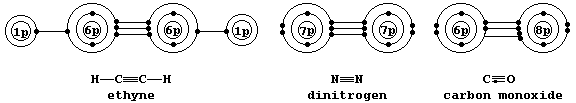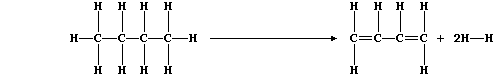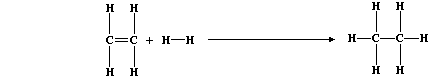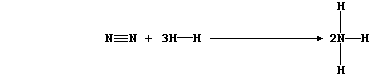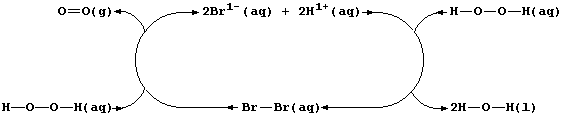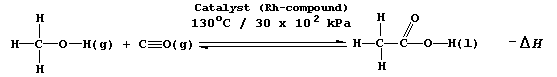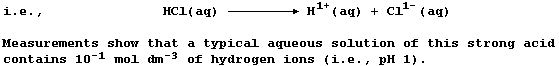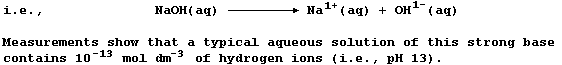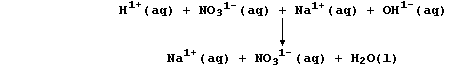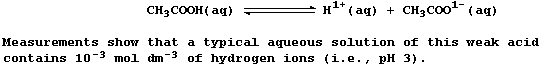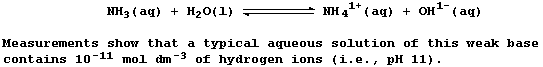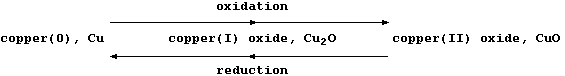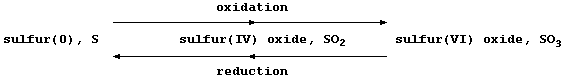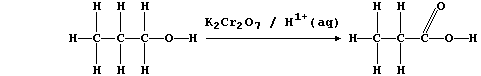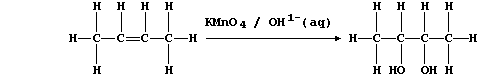AUFBAU1 [SELECTED PRINCIPLES]
[01] INTRODUCTION - STRUCTURE and BONDING (PREAMBLE)
[02] INTRODUCTION - ELECTRONIC STRUCTURES of ATOMS
[03] INTRODUCTION - METALLIC BONDING
[04] INTRODUCTION - LOCALIZED COVALENT BONDING (1)
[05] INTRODUCTION - LOCALIZED COVALENT BONDING (2)
[06] INTRODUCTION - IONIC BONDING
[07] KINETIC THEORY
[08] CHEMICAL ENERGETICS (1)
[09] CHEMICAL ENERGETICS (2)
[10] RATES of REACTIONS (1)
[11] RATES of REACTIONS (2)
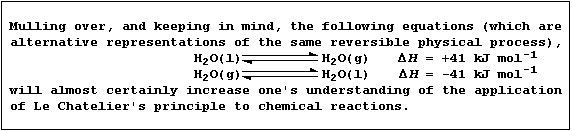
[12] EQUILIBRIA - LE CHATELIER'S PRINCIPLE (1)
[13] EQUILIBRIA - LE CHATELIER'S PRINCIPLE (2)
[14] ACIDS & BASES (1)
[15] ACIDS & BASES (2)
[16] REDOX REACTIONS (1)
[17] REDOX REACTIONS (2)
[18] ELECTROLYSIS (1)
[19] ELECTROLYSIS (2)
[20] ELECTROCHEMICAL CELLS
SELECTED PRINCIPLES: INTRODUCTION - STRUCTURE and BONDING (PREAMBLE)
The intimately related topics of atomic structure and chemical bonding
are universally regarded as the 'heart of Chemistry'; not surprisingly,
therefore, one's knowledge of these topics and this science increase in
parallel. Rather encouragingly, a student and a mature scientist share
similar mental images of both atomic and molecular structure, as can be
illustrated using a common point of reference; thus, ...
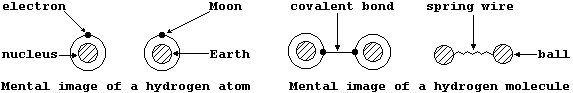
Although neither of these mental images has the slightest relationship
either to physical reality or to their respective analogies, they do
provide a quite convenient starting point from which ever-increasingly
detailed 'pictures' can be drawn ...
The following flow diagram shows a possible pathway to a comprehensive
understanding of either of these two topics: Mental image (student and
mature scientist) ® Sketch 1 (student) ® Sketch 2 ® Finished
drawing ® Water colour ® Oil painting (? ... mature scientist).
Ideally, a student needs to acquire sufficient knowledge, on the one
hand, to be able to draw Sketch 1, and on the other, which will allow
Sketch 2 to be drawn (at a later date) using a rubber and a pencil -
without the need to discard Sketch 1 for a new piece of paper.
For decades, the received wisdom has been that Sketch 1 should be drawn
using theories formulated in the 1910s. In particular, these involve
viewing the electron purely as a particle; and, in terms of presenting
an introduction which is easier to understand, this restrictive view
has undoubted advantages. Nevertheless, the properties of an electron
are consistent with its dual behaviour as a particle and a wave. The
evidence for, and acceptance of, this duality forms the basis of Sketch
2 (and with it comes a spectacular increase in one's understanding of
Chemistry).
In this introduction, apart from one mischievous exception, no attempt
has been made to draw Sketch 1 using contemporary theories. However, a
robust framework is presented which is consistent with two unifying
concepts that have remained unchanged with the advances in theoretical
knowledge: first, electrons occupy either atomic or molecular energy
levels; and second, chemical bonding occurs because the resulting
molecule or compound has a lower energy than its constituent atoms.
Furthermore, because the limitations of Sketch 1 are stated, and the
necessary simplifications are kept to a minimum, it should be possible
to draw Sketch 2 using the same piece of paper.
Energy Levels ... Precepts
Each diagram in Figures (a-h) show an infinite number of steps, each
1.0 m in height, and a 1.0 kg ball which is either occupying a step or
being transferred from one step to another; each step may be correctly
and usefully viewed as an energy level.
Figures (a-h)
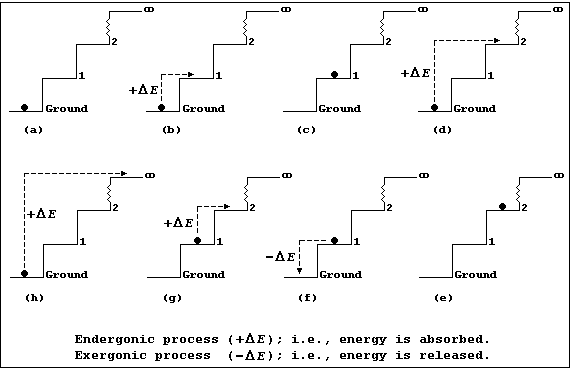
Figure (a) shows a 1 kg ball occupying the ground step; all the other
steps are unoccupied. The energy (E) of the 1.0 kg ball is 0 kJ (i.e.,
E = m × g × h = 1.0 × 10 × 0.0 = 0 kJ).
Figures (b) and (d) show, respectively, the ball being transferred from
the ground to the 1st step and from the ground to the 2nd step; each
process is endergonic (+DE); i.e., energy is absorbed (for example,
by transducing chemical or mechanical energy to potential energy, via
kinetic energy).
Figures (c) and (e) show, respectively, the ball now occupying the 1st
and 2nd steps; it has acquired 10 kJ of potential energy on the 1st
step [Figure (c)] and 20 kJ on the 2nd step [Figure (e)].
Figure (f) shows the ball being transferred back from the 1st to the
ground step: this process is exergonic (-DE); i.e., energy is released
(which, typically, may be as heat and sound energy transduced from
potential energy, via kinetic energy).
Figure (g) shows the ball being transferred from the 1st to the 2nd
step; once transferred it will have acquired another 10 kJ of potential
energy. And, obviously, each similar transfer from a lower to a higher
step (or energy level) will result in the ball acquiring the additional
potential energy.
Figure (h) shows the ball being transferred from the ground step to the
(idealized) infinite step, where the Earth's gravitational force will
be zero; and, here, the ball will no longer be attracted to the Earth.
So, with mischief aforethought, one can say that the hugely endergonic
process of transferring the ball from the ground step to infinity, in
discrete steps, will result in the ball becoming 'ionized'.
And finally, these precepts hold true: firstly, even when the steps are
of non-uniform height (an interesting architectural phenomenon), though
the numerical values would be different; and secondly, in both free and
bonded atoms, where energy levels of non-uniform height tend to be the
rule rather than the exception.
Refreshments ...?
People who are 'positively bursting with the joys of spring' have been
known to express a firmly held opinion: namely, that a swim in ice-cold
water is refreshing after a hot sauna. Presented below is the chemical
equivalent of such a swim: namely, definitions of the commonest terms
used in structure and bonding (a few of these have been simplified).
Matter is anything that occupies space and has mass.
A pure substance is a form of matter that has both definite composition
and distinct properties.
An element is a pure substance which cannot be broken down into simpler
substances by chemical means.
An atom is the smallest particle of an element that can exist and still
retain the ordinary chemical properties of that element.
A proton is a sub-atomic particle, within the nucleus of an atom, which
has unit mass and unit positive charge.
The atomic number (Z) of an element is the number of protons each atom
of that element has in its nucleus; e.g., Z = 17 for chlorine (17Cl).
A neutron is a sub-atomic particle, within the nucleus of an atom,
which has unit mass and zero charge.
The mass number (A) of an atom is the number of protons and neutrons it
has in its nucleus; e.g., A = 35 for a chlorine atom which contains 18
neutrons (35Cl), and A = 37 for one which contains 20 neutrons (37Cl).
Isotopes are atoms of the same element that contain different numbers
of neutrons; e.g., 63Cu and 65Cu are two isotopes of copper.
An electron is a sub-atomic particle, outside the nucleus of an atom,
which has nearly zero mass and unit negative charge.
An ion is an atom (or group of atoms) which has either gained or lost
electrons; e.g., a chloride anion, 17Cl1-, contains 18 electrons (i.e.,
one more than neutral atom, 17Cl0), and a copper(II) cation, 29Cu2+,
contains 27 electrons (i.e., two less than the neutral atom, 29Cu0).
A compound is a pure substance which contains two or more different
elements chemically bonded together in stoichiometric proportions.
A molecule is the smallest part of an element or of a covalently bonded
compound that can exist independently and still retain the ordinary
chemical properties of that element or compound.
A mixture consists of two or more substances which are not chemically
bonded together.
A localized covalent bond describes the mutual electrostatic attraction
of two adjacent nuclei for a shared pair of electrons which occupy the
same molecular energy level.
A delocalized covalent bond describes the electrostatic attraction of
more than two nuclei for a shared pair of electrons which occupy the
same molecular energy level.
An ionic bond describes the electrostatic attraction of two oppositely
charged ions in a crystalline lattice.
SELECTED PRINCIPLES: INTRODUCTION - ELECTRONIC STRUCTURES OF ATOMS
The ground-state electronic structure is the lowest-energy arrangement
of the electrons in each free, gaseous, neutral atom of an element.
Each neutral atom of an element consists of a nucleus of Z protons and
(A - Z) neutrons, together with Z extra-nuclear electrons; where Z is
the atomic number and A is the mass number. The extra-nuclear electrons
are arranged in atomic energy levels; the first four of these can hold
a maximum of 2, 8, 18, and 32 electrons, respectively. The ground-state
electronic structure of an atom is determined by a simple principle:
namely, electrons are arranged in such a way that their total energy is
a minimum. This Table shows two different methods of representing such
electronic structures for all 36 elements in Periods 1 to 4.
|
Occupancies of the
main energy levels |
Occupancies of the sub-levels of
the main energy levels (*) () |
Group |
ZAtom |
1st 2nd 3rd 4th |
1st 2nd 3rd 4th |
1 |
1H |
1 |
1s1 |
18 |
2He |
2 |
1s2 |
1 |
3Li |
2, 1 |
1s2, 2s1 |
2 |
4Be |
2, 2 |
1s2, 2s2 |
13 |
5B |
2, 3 |
1s2, 2s2 2p1 |
14 |
6C |
2, 4 |
1s2, 2s2 2p2 |
15 |
7N |
2, 5 |
1s2, 2s2 2p3 |
16 |
8O |
2, 6 |
1s2, 2s2 2p4 |
17 |
9F |
2, 7 |
1s2, 2s2 2p5 |
18 |
10Ne |
2, 8 |
1s2, 2s2 2p6 |
1 |
11Na |
2, 8, 1 |
1s2, 2s2 2p6, 3s1 |
2 |
12Mg |
2, 8, 2 |
1s2, 2s2 2p6, 3s2 |
13 |
13Al |
2, 8, 3 |
1s2, 2s2 2p6, 3s2 3p1 |
14 |
14Si |
2, 8, 4 |
1s2, 2s2 2p6, 3s2 3p2 |
15 |
15P |
2, 8, 5 |
1s2, 2s2 2p6, 3s2 3p3 |
16 |
16S |
2, 8, 6 |
1s2, 2s2 2p6, 3s2 3p4 |
17 |
17Cl |
2, 8, 7 |
1s2, 2s2 2p6, 3s2 3p5 |
18 |
18Ar |
2, 8, 8 |
1s2, 2s2 2p6, 3s2 3p6 |
1 |
19K |
2, 8, 8, 1 |
1s2, 2s2 2p6, 3s2 3p6 3d0, 4s1
|
2 |
20Ca |
2, 8, 8, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d0, 4s2
|
3 |
21Sc |
2, 8, 9, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d1, 4s2
|
4 |
22Ti |
2, 8, 10, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d2, 4s2
|
5 |
23V |
2, 8, 11, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d3, 4s2
|
6 |
24Cr |
2, 8, 13, 1 |
1s2, 2s2 2p6, 3s2 3p6 3d5, 4s1
|
7 |
25Mn |
2, 8, 13, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d5, 4s2
|
8 |
26Fe |
2, 8, 14, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d6, 4s2
|
9 |
27Co |
2, 8, 15, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d7, 4s2
|
10 |
28Ni |
2, 8, 16, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d8, 4s2
|
11 |
29Cu |
2, 8, 18, 1 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s1
|
12 |
30Zn |
2, 8, 18, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2
|
13 |
31Ga |
2, 8, 18, 3 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p1 |
14 |
32Ge |
2, 8, 18, 4 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p2 |
15 |
33As |
2, 8, 18, 5 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p3 |
16 |
34Se |
2, 8, 18, 6 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p4 |
17 |
35Br |
2, 8, 18, 7 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p5 |
18 |
36Kr |
2, 8, 18, 8 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p6 |
* The superscripts indicate the number of electrons in each sub-level;
for example, 3p5 means that there are 5 electrons in the 3p sub-level.
Introductory courses invariably focus on the occupancies of the
main energy levels; so, the student is well advised to view all the
entries in this column as exotic species of tasty but unripened pears
(... which maybe either plucked at some later date or allowed to rot). |
The ground-state electronic structures of gaseous atoms, as summarized
by their occupancies of the main energy levels, can be represented by
simple electron-structure diagrams (as in the examples shown below).
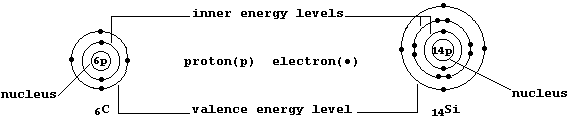
The Table shows the electronic structures of the first 26 elements
placed in the so-called main groups (i.e., 1, 2, and 13 to 18). Apart
from the single exception of helium, the elements in each group contain
the same number of valence (or bonding) electrons in their outermost
energy level; in part, it is this periodicity of electronic structures
which is the basis of the (modern) Periodic Table of the Elements.
The Table also shows the electronic structures of the first 10 elements
placed in Groups 3 to 12. Self-evidently, the occupancies of the two
outermost energy levels show no regularity; but, regrettably, there is
no simple explanation for the observed anomalies. Nevertheless, three
points are worth noting ... First, scandium (Group 3) behaves as if its
electronic structure was effectively 2,8,8,3; and so its chemistry has
some similarity to the elements in Group 13. Second, in contrast to the
main group elements, those in Groups 4 to 11, known as the transition
elements, have valence electrons in the two outermost energy levels or,
more specifically, in the 3d and 4s sub-levels. Because the necessarily
detailed descriptions of these sub-levels are (usually) reserved for an
advanced course, one's initial understanding of the chemistry of the
transition elements will inevitably be quite limited. And third, zinc
(Group 12) has two valence electrons in its outermost energy level; and
so its chemistry has some similarity to the elements in Group 2.
Currently, the received wisdom is that emphasis should be given to the
correlation of the periodicity of electronic structures with chemical
reactivity; an aspect which is readily achieved by limiting studies to
elements of Groups 1, 2, 17, and 18. However, many authors have drawn
attention to a plethora of caveats; just two of these are noted here. §
First, Cotton and Wilkinson have written: "Little of the chemistry of
silicon can be inferred from that of carbon." A particularly important
caveat when one reflects upon two observations: silicon is the second
commonest element in the Earth's crust, but carbon forms more compounds
than any other element apart from hydrogen. The following 'cluette' may
provide some perspective. A student, having compared the occupancies of
the main energy levels, would correctly reason that 'carbon and silicon
should show similar chemistry because they both have the same number of
valence electrons'. Contrastingly, a mature scientist, by drawing upon
advanced theoretical models, would reason that 'their chemistry should
differ because of the different characteristics of their occupied and
unoccupied sub-levels'. Thus, the two individuals will be perceiving
electronic structures (and the Periodic Table) in very different ways.
And second, elements in a group do not always form ions with the same
charge; e.g., in Group 11, the commonest ions of copper, silver, and
gold are, respectively, Cu(II), Ag(I), and Au(III) - admittedly, these
are transition metals: but then so are over half of all those known.
§ See, for example, F. A. Cotton and G. Wilkinson, Advanced Inorganic
Chemistry, Wiley, New York, 1988; and J. E. Huheey, Inorganic Chemistry
(Chapter 17), Harper Row, New York, 1983.
SELECTED PRINCIPLES: INTRODUCTION - METALLIC BONDING
An element (E) is defined as 'a pure substance which cannot be broken
down into simpler substances by chemical means'. Of the 111 elements so
far characterized, 81 are stable (H ... Mo, Ru ... Nd, and Sm ... Bi),
whereas 30 occur only as radioactive isotopes (Tc, Pm, and Po ... Uuu);
extensive compilations of various data for most elements are included
in reference books (e.g., The Handbook of Physics and Chemistry).
This first Table shows selected data for 13 of the 81 stable elements,
including those for the best electrical conductor (silver).
|
CE /
MW cm-¹ |
CT /
W m-¹ K-¹ |
TM /
K |
TB /
K |
r
g cm-³ |
DH1 /
kJ mol-¹ |
DH2 /
kJ mol-¹ |
Ag |
68.0 |
428 |
1235 |
2433 |
10.5 |
289 |
731 |
Cu |
64.5 |
403 |
1356 |
2853 |
8.9 |
339 |
745 |
Au |
48.8 |
319 |
1337 |
3080 |
19.3 |
369 |
890 |
Al |
40.0 |
236 |
933 |
2623 |
2.7 |
314 |
578 |
Mg |
25.4 |
157 |
922 |
1363 |
1.7 |
150 |
938 |
Na |
23.8 |
142 |
371 |
1173 |
0.97 |
109 |
496 |
In |
12.5 |
84 |
430 |
2323 |
7.3 |
244 |
558 |
Pt |
10.2 |
72 |
1995 |
3993 |
21.5 |
565 |
868 |
Ga |
7.4 |
41 |
302 |
2342 |
5.9 |
289 |
579 |
As |
3.8 |
39 |
886-subl. |
5.7 |
290 |
946 |
Hg |
1.1 |
8 |
234 |
630 |
13.6 |
61 |
1007 |
Mn |
0.7 |
8 |
1517 |
2393 |
7.4 |
279 |
717 |
Te |
0.001 |
- |
723 |
1263 |
6.3 |
199 |
869 |
Electrical conductivity (CE); Thermal conductivity (CT); Melting point
(TM); Boiling point (TB); Density (r) at 293 K. Heat of sublimation
(DH1), defined as the energy required to separate a gaseous atom from
the solid element; i.e., E(s) ® E(g). First ionization energy (DH2),
defined as the energy required to remove one electron from an isolated
gaseous atom; i.e., E(g) ® E1+(g) + 1e-. Conductivity values refer
to data obtained at ambient temperatures (288 - 298 K). |
These compilations show that no element has an electrical conductivity
between 0.001 MW cm-¹ (Te) and 0.7 MW cm-¹ (Mn), and so a metallic
element can be precisely defined as 'a pure substance which cannot be
broken down into simpler substances by chemical means, and which has an
electrical conductivity greater than or equal to 0.7 MW cm-¹ at ambient
temperatures'. This definition may appear unduly pedantic, but its use
results in 62 of the 81 stable elements being classified as metallic
and 19 as non-metallic (H, He, B, C, N, O, F, Ne, Si, P, S, Cl, Ar, Se,
Br, Kr, Te, I, and Xe). Furthermore, this definition tacitly recognizes
the occurrence of elements whose conductivities are increased either by
photo- or thermal-excitation or by the presence of trace impurities
(e.g., 'semi-conductors' such as silicon), as well as those which show
'superconductivity' at very low temperatures (e.g., hydrogen).
Three initial problems arise when attempting to introduce an overview
of metallic elements. First, they do show typical properties, such as
high electrical and thermal conductivities, high melting and boiling
points, and high densities: but there are several anomalies which are
difficult to explain; e.g., the melting points of caesium, gallium,
mercury, potassium, rubidium, and sodium are all lower than several
non-metallic elements. Second, there is a reasonably acceptable linear
relationship between electrical and thermal conductivities (i.e.,
CE » k × CT + c): but, as is clearly apparent from inspection of those
data in the above Table, there are no correlations between any other
pair of physical quantities. And third, their typical properties are
invariably attributed to metallic bonding: but simple descriptions of
this phenomenon, which are not misleading, have proven elusive ...
The following description of a metal, or minor variants thereof, has
been commonly included in introductory texts: 'a metal consists of a
lattice of positive ions embedded in a sea of electrons'. However, for
at least three reasons, this description must be firmly eschewed.
First, the term 'lattice' refers strictly to the solid state: but, as
evinced by conductivity data (see, for examples, those shown in this
second Table), metallic bonding clearly persists in the liquid state.
|
Hg |
Cs |
Ga |
Rb |
K |
Na |
Conductivity at 293 K / MW cm-¹ |
1.0 |
5.0 |
7.5 |
8.0 |
16.5 |
24.0 |
Melting point / K |
234 |
301 |
303 |
312 |
336 |
371 |
Conductivity at 373 K / MW cm-¹ |
1.0 |
2.0 |
3.5 |
3.5 |
5.5 |
10.5 |
Second, researchers do not invoke 'ionization' in their descriptions of
metals; the experimental data, together with theoretical calculations,
support a bonding model which is fundamentally covalent in character. *
And third, 'a sea of electrons' is a peculiarly mixed metaphor; thus,
conduction in aqueous solutions occurs via free-moving ions, whereas
conduction in metals occurs via free-moving delocalized electrons. #
Quite reasonably, one would expect, as a minimum, a description of the
bonding in metallic elements to provide at least partial explanations
for two observations: first, the 100-fold difference in electrical
conductivity between silver and manganese; and second, the 700-fold
difference in electrical conductivity between manganese and tellurium.
Unfortunately, however, such expectations probably cannot be realized
from a simple qualitative description of metallic bonding. Furthermore,
there may not be such a description which is illuminating without being
misleading: so the following attempt may only score marks 'for effort'.
'A metal consists of a giant covalent structure, in which each atom has
contributed one or more of its valence electrons to the formation of
omnidirectional, delocalized covalent bonds that extend throughout the
structure.' §
Strictly speaking, this description of a metal does not 'explain' its
characteristic properties: but advanced theories of metallic bonding
indicate that it is concordant. In particular, it is consistent with
two platitudes: 'a metal is a good conductor of electricity because of
the presence of free-moving delocalized electrons', and 'a non-metal is
a poor conductor of electricity because of the absence of free-moving
delocalized electrons'.
* See, for example, R. Hoffmann, Solids and Surfaces, VCH Publishers,
New York, 1988 (and references cited therein).
# An extended critique of this description would be inappropriate here
and, indeed, unnecessary because a beautifully balanced summary of both
historical and contemporary bonding theories in metals has been written
by J. D. Lee [Concise Inorganic Chemistry (pp. 114 - 118), Harper Row,
London, 1991]. However, the student is invited to consider constructing
and testing various hypotheses, using the electrical conductivity, heat
of sublimation, and first ionization data shown in the first Table.
§ The following notes should provide a slightly deeper perspective ...
Each sodium atom has one electron in its outermost atomic energy level:
so 1 mole of sodium metal contains 6.022 × 10²³ valence electrons which
contribute to the formation of covalent bonds. A covalent bond contains
two electrons which occupy the same molecular energy level: so 1 mole
of electrons contains half this number of covalent bonds. In principle,
each of these (very closely spaced) levels encompasses the 6.022 × 10²³
nuclei: so there are 3.011 × 10²³ omnidirectional, delocalized covalent
bonds extending throughout 1 mole of sodium metal.
SELECTED PRINCIPLES: INTRODUCTION - LOCALIZED COVALENT BONDING (1)
Bonding between atoms, which occurs because the resulting molecule or
compound has a lower energy than its constituent atoms, is achieved by
redistributing the valence (or bonding) electrons. In covalent bonding,
this redistribution occurs by the atoms sharing two or more electrons.
The term localized covalent bond describes the mutual electrostatic
attraction of two adjacent nuclei for a shared pair of electrons which
occupy the same molecular energy level.
The Mechanism of Covalent Bonding
Consider two hydrogen atoms, Ha and Hb. The (potential) energy of each
electron, in its own separate atomic energy level, is reduced when both
electrons enter a molecular energy level which encompasses both nuclei;
in doing so, and forming a localized covalent bond, potential energy is
transduced to heat energy. So, bond formation is an exothermic process:
Ha(g) + Hb(g) ® HH(g) DH = -432 kJ mol-¹ *
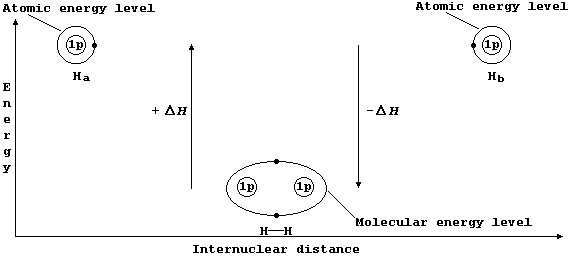
Localized Single Bonds
The sketch graph above includes a method of representing the electronic
structure of dihydrogen which is a fairly reasonable approximation to
physical reality; in particular, it emphasizes that - after bonding -
the two electrons are indistinguishable from one another and that they
occupy one molecular energy level (not separate atomic energy levels).
However, similar representations for more complex molecules are rarely
used because the resulting diagrams are inevitably more complicated.
Shown below, for dihydrogen, is the simplest type of electron-structure
diagram.
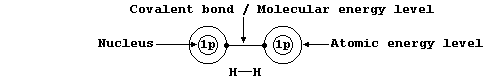
The merits of this type of electron-structure diagram are two-fold.
First, the retention of the atomic energy levels simplifies 'electronic
book-keeping'. And second, there is a clear and direct relationship to
one standard method of representing a molecule: i.e., by its structural
formula.
* Conversely, bond breaking, as in the dissociation of dihydrogen, is
an endothermic process;
HH(g) ® Ha(g) + Hb(g) DH = +432 kJ mol-¹
Shown below are electron-structure diagrams for atomic fluorine and
molecular fluorine (or difluorine).

A fluorine atom contains seven electrons in its outermost atomic energy
level; because one of these is unpaired, such an atom is known as a
free-radical. The potential energy of this unpaired electron is reduced
when it becomes part of a covalent bond; as occurs, for example, when a
molecule of difluorine is formed.
The covalent bond in difluorine is formed in the same manner as that in
dihydrogen. Thus, one electron from the outermost atomic energy level
of each fluorine atom enters a molecular energy level which encompasses
both nuclei; the other (non-bonding) electrons form lone-pairs.
Difluorine is an example of a molecule which conforms to the so-called
'octet rule'; i.e., an atom - other than H, He, Li, Be, or B - tends to
form bonds until it is surrounded by eight valence electrons. [This is
a useful rule of thumb for the electronic structures of molecules which
contain only carbon, nitrogen, oxygen, or fluorine. Nevertheless, there
are innumerable examples of compounds which contain bonded atoms that
have either an 'incomplete' or an 'expanded' octet.] *
Shown below are electron-structure diagrams for hydrogen fluoride and
water.

Because the bonds in these two molecules are formed in the same manner,
and for the same reason, as both dihydrogen and difluorine, one might
expect no further comment. But, together, these four molecules beg an
important question: are all covalent bonds the same? The short answer
is no! However, in an introductory text, quite how much detail should
accompany this flat answer is difficult to judge; and so, rather than
throwing caution to the winds, only three brief points are made here.
First, by analogy with the different energy levels observed in atoms,
one would expect there to be different molecular energy levels. Indeed,
although well beyond the scope of this text, both spectroscopic studies
and theoretical calculations provide support for such an expectation;
e.g., the molecular energy levels associated with the covalent bonds in
H-H, F-F, H-F, and H-O-H are all different.
Second, perhaps intuitively, one might expect that the electrons are
not equally distributed when the two nuclei are different; the unequal
distribution of 'electron density' is known as polarization (and occurs
in both H-F and H-O-H, for example: but not in either H-H or F-F).
And third, quantitative determinations of molecular energy levels and
polarization, amongst other bonding parameters, allow many reactions of
molecules to be both correlated and predicted.
* As a caveat to the 'octet rule', consider the following. Each of the
46 chromosomes present in (most) cells of the human body contain bonded
phosphorus which does not adopt the electronic structure of argon. [...
And setting aside identical sprogs, the exponentially increasing human
population is? ... And setting aside the depressing fact that numbers
are ever decreasing, because of pollution, the number of species is?]
SELECTED PRINCIPLES: INTRODUCTION - LOCALIZED COVALENT BONDING (2)
Multiple bonding will occur between atoms if the resulting molecule or
compound has a lower energy than that achieved by single bonding. *
Localized Double Bonds
Shown below are electron-structure diagrams for ethane and ethene.
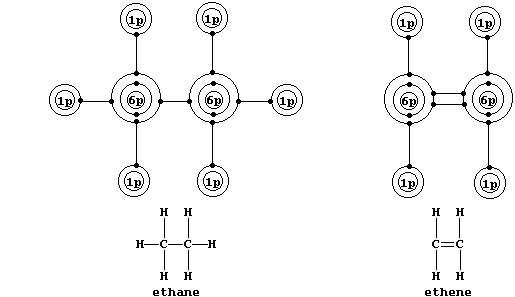
Ethene contains what is (universally) known as a double bond. However,
perhaps contrary to one's initial expectations, these two bonds are not
identical: one of these, known as a s (sigma) bond, consists of two
electrons at one molecular energy level; and the other, known as a p
(pi) bond, consists of two electrons at another molecular energy level.
These equations summarize the partial dissociation of ethene (Eq. 1)
and the complete dissociation of ethene (Eq. 2) and ethane (Eq. 3):
Eq. 1 H2C=CH2 ® H2C-CH2 DH = +264 kJ mol-¹
Eq. 2 H2C=CH2 ® H2C + CH2 DH = +610 kJ mol-¹
Eq. 3 H3C-CH3 ® H3C + CH3 DH = +346 kJ mol-¹
Equations 2 and 3 show that the double bond is stronger than the single
bond by 264 kJ mol-¹; and Equations 1 and 3 show that the p bond is
weaker than the s bond by 82 kJ mol-¹. Despite the increased strength
imparted by double bonding, ethene is more reactive than ethane; e.g.,
in the absence of light, bromine reacts with ethene but not ethane. #
A partial explanation for this apparent anomaly is as follows: both
molecules contain a s bond between the carbons, but bromine reacts with
the (localised) p bond present only in ethene.
* No attempt will be made here to explain (in detail) the reasons why
some pairs of atoms have little or no tendency to form multiple bonds;
e.g., in contrast to the vast numbers of compounds which contain C=C
bonds, those with Si=Si bonds are very rare indeed.
# Demonstrating the differences in chemical reactivity between ethane
and ethene is cumbrous, because both are gases at r.t.p.; the simplest
homologues of the alkanes (CnH2n+2) and alkenes (CnH2n) which are not
either gases or highly volatile liquids, and which can be investigated
safely and conveniently, are hexane and hex-1-ene, respectively.
Localized Triple Bonds
Shown below are electron-structure diagrams for ethyne, dinitrogen, and
carbon monoxide.
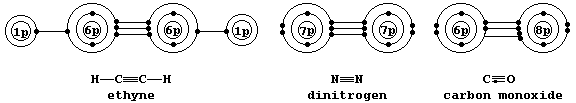
Each of these molecules contains what is known as a triple bond; i.e.,
one s bond and two p bonds (p1 and p2), with each bond consisting of
two electrons in different molecular energy levels that encompass the
nuclei of the principal atoms (i.e., C, N, and O). Although each bonded
principal atom contains an octet of electrons, the different manner by
which this is achieved in carbon monoxide merits describing the bonding
in detail. Setting aside each lone-pair, localized about its respective
nucleus, a description of the triple bonding in carbon monoxide is as
follows: one s bond, containing one electron from each atom; one p bond
(p1), containing one electron from each atom; and another p bond (p2),
containing two electrons from the oxygen atom.
|
Nominal bond energy of
each bond / kJ mol-¹ |
Total bond energy
/ kJ mol-¹ |
|
s |
p1 |
p2 |
S s + p1 + p2 |
Ethyne (HCºCH) |
346 |
264 |
205 |
815 |
Dinitrogen (NºN) |
167 |
251 |
528 |
946 |
Carbon monoxide (CºO) |
360 |
385 |
324 |
1069 |
The above Table reveals that each of these triple bonds is very strong
but that the strength of each component varies within and between the
three molecules. Their (relative) difficulty of reduction by dihydrogen
is NºN >> CºO > HCºCH; an order that parallels the energy of the p2 bond
(NºN >> CºO > HCºHC), but not the total bond energy (CºO > NºN > HCºCH).
This correlation may just be coincidental, however; certainly, other
bonding parameters, including molecular energy levels and polarization,
are known to influence the rate of reaction. #
* A covalent bond in which one of the atoms donates both electrons is
called either a coordinate or a dative bond; such bonds are often, but
not always, represented by an arrow showing the direction of donation.
Coordinate bonding is very common (particularly so in compounds of the
transition elements); one example is an ammonium ion, in which nitrogen
uses its lone-pair to form a coordinate bond with a hydrogen ion.
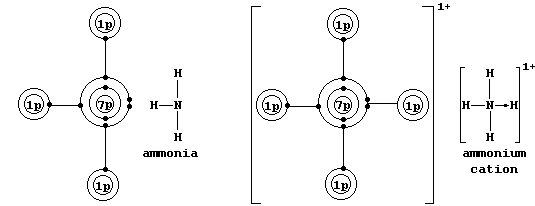
# Here, for example, it is noteworthy that dinitrogen is non-polar,
whereas carbon monoxide and ethyne are both polar molecules (with an
unequal distribution of 'electron density' about the nuclei).
SELECTED PRINCIPLES: INTRODUCTION - IONIC BONDING
Bonding between atoms, which occurs because the resulting molecule or
compound has a lower energy than its constituent atoms, is achieved by
redistributing the valence (or bonding) electrons. In ionic bonding,
this redistribution occurs by the atoms transferring one or more
electrons. The term ionic bond describes the electrostatic attraction
of two oppositely charged ions in a crystalline lattice.
The Mechanism of Ionic Bonding
Formally, the ionic compound sodium fluoride - which contains Na1+ and
F1- ions, each with an octet of electrons - results from the transfer
of one valence electron from a sodium atom to a fluorine atom; i.e.,
Na atom + F atom ® Na1+ion + F1-ion
2,8,1 2,7 2,8 2,8
Despite the attractiveness of such a simple summary, one needs to peer
into the mechanism of ionic bonding in order to prevent misconceptions.
The formation of solid sodium fluoride, NaF(s), from solid sodium and
gaseous difluorine, can be divided into five ergonic processes; i.e.,
Na(s) ® Na(g) DH1
Na(g) ® Na1+(g) + 1e- DH2
F2(g) ® 2F(g) DH3
F(g) + 1e- ® F1-(g) DH4
Na1+(g) + F1-(g) ® Na1+(s)F1-(s) DHL
Experiments show that formation of these gaseous ions is endothermic;
i.e., SDHI, the value of DH1 + DH2 + DH3 + DH4, is positive. In sharp
contrast, formation of the solid from these gaseous ions is exothermic;
i.e., the value of DHL, known as the lattice energy, is negative. Solid
sodium fluoride forms because the value of DH, DHL - SDHI, is negative;
so, the 'driving force behind' the formation of sodium fluoride is the
overwhelmimg exothermicity of the attractions of the oppositely charged
ions for each other (... and not the formation of ions with an octet).
Furthermore, because experiments have shown that, for every combination
of metallic and non-metallic element, the corresponding value of SDHI
is positive, the formation of an ionic compound is determined largely
by the value of DHL (the lattice energy).
This ('long-winded') approach above allows a qualitative understanding
of some apparent anomalies; two illustrative examples are given here.
Firstly, Na2O, MgO, Al2O3, NaCl, and MgCl2 are all ionic compounds in
which the ions have an octet: but, although aluminium and chlorine
can readily form ions with an octet, aluminium chloride is a covalent
compound. * The explanation is as follows: DHL - SDHI is negative in
each of the first five compounds, but DHL - SDHI is positive for the
hypothetical ionic compound Al3+(s)3Cl1-(s)
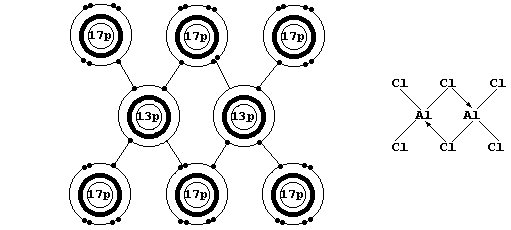
* When gaseous aluminium chloride is cooled, discrete AlCl3 molecules
dimerize to form Al2Cl6 (see the electron-structure diagram above).
And secondly, NaCl and MgCl2 are both ionic compounds in which the ions
have an octet: but, although transition elements do not form ions with
an octet, CuCl2 and FeCl2 are also ionic. The explanation is as before;
thus, DHL - SDHI is negative in each of these four compounds.
In the absence of either experimental or calculated data, the student
will ask (or even demand) to know how one predicts the type of bonding
in any given compound. Although there is no predictive method which is
foolproof, two rules of thumb are useful: first, the bonding between
non-metallic elements is normally covalent; and second, the bonding
between metallic and non-metallic elements is commonly ionic. *
The Structures and Physical Properties of Ionic Compounds
The diagram below shows the structure of NaCl; the oppositely charged
ions are arranged in a symmetrical pattern within a crystal lattice.
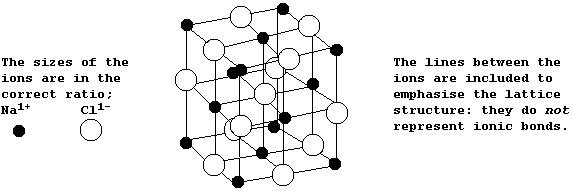
The electrostatic attractions between the oppositely charged ions in
the crystal lattices of ionic compounds are strong and omnidirectional:
and so, such compounds show several characteristic physical properties;
however, only three of these will be considered here.
First, they tend to have high melting points; a large amount of thermal
energy must be supplied to the crystal lattice before the ions vibrate
vigorously enough to overcome the electrostatic forces of attraction.
Second, they do not conduct electricity in the solid state, because
there are no free-moving ions to carry the current: contrastingly, they
do conduct electricity in either the liquid state or when dissolved in
water, because the ions are free to move.
And third, they tend to be insoluble in organic solvents and soluble in
water; the hydration energy released when ions are dissolved in water
is often similar to or greater than the lattice energy.
Not surprisingly, these characteristic physical properties are used as
evidence for ionic bonding: but there are at least three caveats.
First, high melting points are also observed for covalent substances
with giant structures [e.g., carbon-graphite and silicon(IV) oxide]. #
Second, the measurement of electrical conductivity can be precluded
because the compound either thermally decomposes before melting or has
a low solubility in water [e.g., many carbonates and nitrates decompose
before melting, and most oxides and sulfides are insoluble in water].
And third, a wide variety of covalent compounds are also soluble in
water [e.g., many alcohols and carbohydrates with small molar masses].
* In point of fact, the exceptions to this rule of thumb are legion.
However, such exceptions usually make no more than 'guest appearances'
in an introductory course (... a rare case of 'ignorance is bliss' ?).
# Typically, covalent substances with simple molecular structures have
low melting points, because the attractive forces between molecules are
weak, are insulators of electricity, because there are no free-moving
ions, and are soluble in organic solvents such as trichloroethane.
SELECTED PRINCIPLES: KINETIC THEORY
Diffusion, the movement of particles from a high to low concentration,
and Brownian Motion, the random movement of solid particles suspended
in a liquid or in a gas, are two pieces of evidence which provide the
basis for the kinetic particulate theory of matter - a model which is
used to explain various physical properties of substances.
The main points in the kinetic theory of gases can be summarized as
follows. One, a gas consists of particles in ceaseless random motion.
Two, the volume of the particles themselves is negligible in comparison
with the total volume of the gas. Three, the particles exert neither
attractive nor repulsive forces on one another. Four, the particles
make elastic collisions; i.e., no energy is lost in collisions between
the particles. And five, the average kinetic energy of the particles is
proportional to the absolute temperature of the gas.
Strictly speaking, the kinetic theory refers only to gases. However,
because the difference between the three states of matter is to do with
the amount of energy each state has (i.e., gas > liquid > solid), parts
of the theory are used to provide a model of each of these states; the
Table below summarizes their characteristic properties.
|
SOLIDS |
LIQUIDS |
GASES |
Kinetic energy of particles |
None |
Low |
High |
Motion of particles |
Vibrate about
fixed positions |
Freely around
one another |
Ceaseless
and random |
Rate of diffusion |
Very slow |
Slow |
Fast |
Attractive forces |
Strong |
Fairly weak |
Negligible |
Spacing of particles |
Close contact |
Close contact |
Far apart |
Arrangement of particles |
Regular |
Random |
Random |
Volume |
Fixed |
Fixed |
Variable |
Shape |
Fixed |
Variable |
Variable |
Compressibility |
Virtually
incompressible |
Only slightly
compressible |
Very
compressible |
Density |
High |
High |
Very low |

1. The average kinetic energy, and so the average speed, of molecules
in a gas increases with temperature. Although the derivation of any of
the mathematical equations associated with the kinetic theory of gases
is beyond the scope of this text, advanced studies do show that the
average speed of gas molecules can be calculated using this equation,
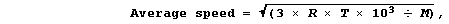
where: R is a constant, with a value of 8.314 J K-¹ mol-¹; T is the
temperature (in K); and, M is the molar mass (in g mol-¹).
Complete this Table, using the equation above to calculate the average
speed of the molecules of these gases at 723 K (450°C).
|
Average speed of molecules / m s-¹ |
Gas |
M / g mol-¹ |
at 298 K |
at 373 K |
at 723 K |
Dihydrogen (H2) |
2 |
1928 |
2157 |
|
Methane (CH4) |
16 |
682 |
763 |
|
Ammonia (NH3) |
17 |
661 |
740 |
|
Carbon monoxide (CO) |
28 |
515 |
576 |
|
Dinitrogen (N2) |
28 |
515 |
576 |
|
Ethene (C2H4) |
28 |
515 |
576 |
|
Ethane (C2H6) |
30 |
498 |
557 |
|
Dioxygen (O2) |
32 |
482 |
539 |
|
Propane (C3H8) |
44 |
411 |
460 |
|
Butane (C4H10) |
58 |
358 |
401 |
|
Sulfur dioxide (SO2) |
64 |
340 |
381 |
|
[7]
2. In everyday-life, water is the substance most commonly observed in
its three physical states (usually known as ice, water, and steam); the
diagram below shows the processes accompanying the physical changes.
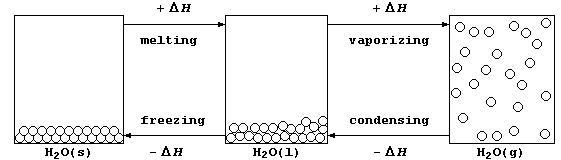
(a) Calculate the heat energy (H) needed to melt one mole of ice at
273 K. [Use the equation H = m × l, where: m is the mass of substance;
and, l is the specific latent heat of fusion of ice, with a value of
0.334 kJ g-¹.] ________________________________________________________
(b) Calculate the heat energy (H) needed to increase the temperature of
one mole of water from 273 to 373 K. [Use the equation H = m × c × DT,
where: m is the mass of substance; c is the specific heat capacity of
water, with a value of 0.00420 kJ g-¹ K-¹; and, DT is the temperature
rise.] ________________________________________________________________
(c) Calcuate the heat energy (H) needed to vaporize one mole of water
at 373 K to steam. [Use the equation H = m × l, where: m is the mass
of substance; and, l is the specific latent heat of vaporization of
water, with a value of 2.26 kJ g-¹.] __________________________________
(d) State the total amount of heat energy released to the surroundings
when one mole of steam at 373 K is converted to ice at 273 K. _________
_______________________________________________________________________
[7]
3. Name the process in which molecules go directly from the solid
into the vapour phase; as is observed, for example, with carbon dioxide
[solid ® gas]. ___________________________________________________
[1]
SELECTED PRINCIPLES: CHEMICAL ENERGETICS (1)
During a chemical reaction, bonds of the reactants are broken and then
those of the products are formed. Bond breaking is an endothermic or,
more generally, an endergonic process: by contrast, bond forming is an
exothermic or, more generally, an exergonic process. Each bond type has
its own bond energy, defined as 'the amount of energy needed to break
one mole of the bond or released when one mole of the bond is formed';
some typical values are shown in the Table immediately below.
Bond type |
O-O |
C-C |
C-H |
H-H |
O-H |
O=O |
C=C |
CºC |
Bond energy / kJ mol-¹ |
146 |
346 |
413 |
432 |
463 |
497 |
610 |
815 |
The heat energy change (DH) for a chemical reaction can be calculated
using bond energies - as these two exemplars illustrate.
The equation for the combustion of dihydrogen can be written as:
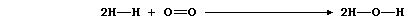
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
2 H-H |
864 |
4 O-H |
1852 |
1 O=O |
497 |
|
|
Total = 1361 |
Total = 1852 |
Energy absorbed - energy released = 1361 - 1852 = DH -491 kJ mol-¹.
Thus, in an exothermic reaction, 491 kJ of energy are released to the
surroundings when 2 moles of dihydrogen combust; i.e., 245.5 kJ mol-¹
of dihydrogen. [This combustion is most familiar as the explosive 'pop'
observed when testing for hydrogen gas with a lighted splint.]
The equation for the dehydrogenation of ethane can be written as:

Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
6 C-H |
2478 |
4 C-H |
1652 |
1 C-C |
436 |
1 C=C |
610 |
|
|
1 H-H |
432 |
Total = 2824 |
Total = 2694 |
Energy absorbed - energy released = 2824 - 2694 = DH +130 kJ mol-¹.
Thus, in an endothermic reaction, 130 kJ of energy are absorbed from
the surroundings when 1 mole of ethane dehydrogenates. [This reaction,
incidentally, is one of the principal methods by which dihydrogen is
obtained on an industrial scale.]

1. Dehydrogenation reactions are used in industry to obtain a range of
chemicals. Butadiene, for example, can be obtained by dehydrogenation
of butane; the equation for this reaction can be written as:
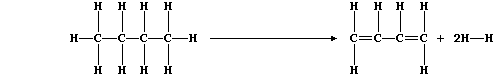
(a) Use the appropriate bond energies to complete this Table,
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total = |
Total = |
[4]
in order to show that the heat energy change (DH) for this endothermic
reaction is +260 kJ mol-¹. ____________________________________________
_______________________________________________________________________
[1]
(b) Butadiene is a monomer used to synthesize various polymers; these,
in turn, are used to manufacture a variety of synthetic rubbers (known
as elastomers). Suggest the chemical name of one polymer derived from
butadiene. ____________________________________________________________
[1]
2. Hydrogenation reactions are, unsurprisingly, the chemical opposites
of dehydrogenation reactions. Ethane, for example, can be obtained by
hydrogenation of ethene; the equation for this reaction can be written
as: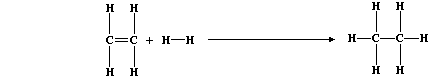
Use the appropriate bond energies to complete this Table,
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
|
|
|
|
|
|
|
|
|
|
|
|
Total = |
Total = |
[2]
in order to show that the heat energy change (DH) for this exothermic
reaction is -130 kJ mol-¹. ____________________________________________
_______________________________________________________________________
[1]
3. The hydrogenation of ethene to ethane is an exothermic reaction
with DH of -130 kJ mol-¹: whereas, the dehydrogenation of ethane to
ethene is an endothermic reaction with DH of +130 kJ mol-¹. This
'mirror-symmetry' is consistent with the Principle of the Conservation
of Energy, which states that 'energy is neither created nor destroyed,
but can be transduced from one form to another'. Noting that DH for the
dehydrogenation of butane to butadiene is +260 kJ mol-¹, what must
be DH for the hydrogenation of butadiene to butane? __________________
[1]
SELECTED PRINCIPLES: CHEMICAL ENERGETICS (2)
Regardless of whether a chemical reaction is exothermic or endothermic,
energy is required to break bonds of the reactants before those of the
products are formed. So, for a reaction to occur, the particles must
collide with sufficient energy for these bonds to be broken; this
minimum energy is known as the activation energy.
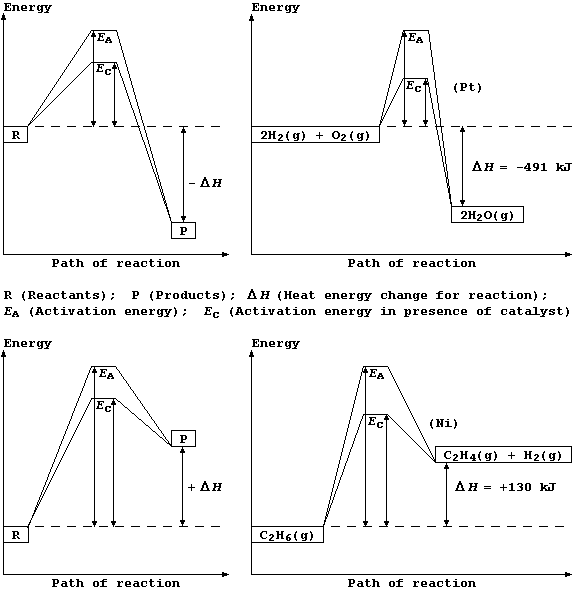
Shown below are two pairs of energy level diagrams: the first shows the
the general characteristics and an example of an exothermic reaction;
and the second shows the general characteristics and an example of an
endothermic reaction.

1. Hydrogenation is often used to convert unsaturated compounds, which
contain carbon double or triple bonds, into saturated compounds, which
contain carbon single bonds. The symbol equation for the Ni-catalyzed
hydrogenation of ethene to (saturated) ethane is:
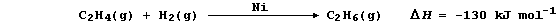
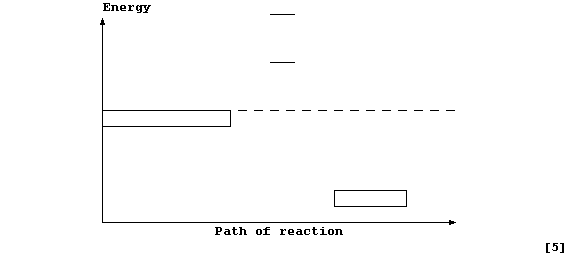
Complete and label this energy level diagram for the above reaction.
Energy
___
___
_________________ _ _ _ _ _ _ _ _ _ _ _ _ _
_________________
_________
_________
___________________________________________
Path of reaction
[5]
An important commercial application of hydrogenation is that used in
the manufacture of margarine, where unsaturated vegetable oils are
converted into saturated fats. The rate of this conversion is increased
by the use of a Ni-catalyst, which reduces the activation energy, and a
fairly high temperature (170°C), which increases the kinetic energy of
the particles so that more have the required activation energy for
successful collisions. Suggest one disadvantage of using an even higher
reaction temperature. _________________________________________________
[1]
Name two vegetable oils used in the manufacture of margarine. _________
_______________________________________________________________________
[2]
2. Hydrogenation is but one of many examples of chemical reduction;
another is that of dinitrogen to ammonia: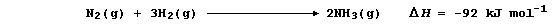
This equation can be written as:
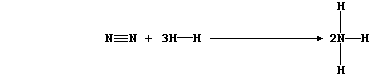
Show that the heat energy change (DH) for the above exothermic reaction
is -92 kJ mol-¹, by completing the Table using these bond energies:
946 kJ mol-¹ (NºN); 432 kJ mol-¹ (H-H); and 389 kJ mol-¹ (N-H).
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
|
|
|
|
|
|
|
|
Total = |
Total = |
[3]
State the amount of heat energy released to the surroundings when
1 mole of ammonia is formed. __________________________________________
[1]
SELECTED PRINCIPLES: RATES OF REACTIONS (1)
During any chemical reaction the concentrations of reactants decrease
and the concentrations of products increase. The rate of reaction is
the change in the concentration of reactants or products with time;
and this change can be determined by measuring any convenient chemical
or physical property of the reaction mixture (e.g., colour intensity,
electrical conductivity, mass, pH, precipitate formation, temperature,
or volume of gas).
Independent variables known to affect the rates of reactions include
concentration, pressure, surface area, the presence of an inhibitor,
temperature, light, and the presence of a catalyst. The effect of these
particular variables on reaction rates can be explained qualitatively
by the use of collision theory; i.e., particles must collide before
they react, and that a reaction will only occur if the colliding
particles contain more than a certain minimum amount of energy - known
as the activation energy. Thus, ...
An increase in concentration, or in pressure (for gases), increases the
number of collisions between the particles.
An increase in the surface area of reactants, which are either solids
or immiscible liquids, increases the number of particles exposed to
collisions.
An inhibitor decreases the number of collisions between the particles,
effectively by reducing either the concentration or the surface area
of reactants or catalyst.
An increase in temperature results in an increase in the kinetic energy
of the particles, so more will have the required activation energy for
successful collisions.
Light energy absorbed by reactants is transduced into chemical energy,
so more of these particles will have the required activation energy for
successful collisions.
A catalyst decreases the activation energy of a reaction, so more
particles will have the required energy for successful collisions.
Quantitative investigations measuring rates of reactions follow the
standard precepts of controlled experiments ... First, a hypothesis is
formulated, which states a predicted relationship between a dependent
variable and one independent variable. Second, values of the dependent
variable are measured as values of the chosen independent variable are
changed systematically, whilst all other variables are both measured
and held constant (so as 'to isolate the independent variable'). And
third, the data are examined critically to determine whether there is
evidence to support the formulated hypothesis; this examination must
include an assessment of the various sources of experimental error.
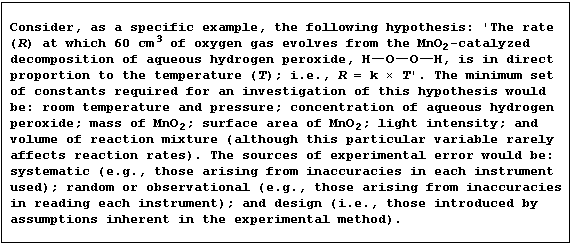
1. The equation for the decomposition of aqueous hydrogen peroxide is:
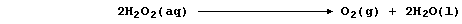
This first sketch graph shows the effect of changing light intensity on
the rate of decomposition of aqueous hydrogen peroxide.
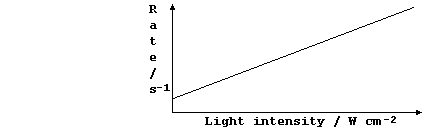
These four sketch graphs show the effects, on the rate of decomposition
of aqueous hydrogen peroxide, of the following independent variables:
concentration of a soluble inhibitor; volume of reaction mixture; type
of catalyst; and, temperature of reaction mixture in the presence of
the enzyme catalase. Label each graph with the correct independent
variable and a suitable unit.
2. In order to confirm the catalytic activity of a solid substance,
what two measurements of the substance need to be made (apart from
measuring increases in reaction rate)? ________________________________
_______________________________________________________________________
[2]
3. What physical property of a solvent usually determines the minimum
temperature for a reaction carried out in solution? ___________________
[1]
4. As a 'rule of thumb', the rate of a chemical reaction doubles for
every 10°C increase in temperature. If the rate of a certain chemical
reaction was 32 s-¹ at 40°C, what would be its rate at 0°? __________
_______________________________________________________________________
_______________________________________________________________________
[2]
5. Name two refrigerants used to preserve biological materials such
as reproductive cells. ________________________________________________
[2]
6. Visible light is a region of the electromagnetic spectrum (e.m.s).
Name two other regions of the e.m.s. which might increase the rate of
certain chemical reactions. ___________________________________________
[2]
SELECTED PRINCIPLES: RATES OF REACTIONS (2)
Until relatively recently, catalysts had acquired the status of being
merely 'wizard's potions', with a particular potion increasing the rate
of a certain chemical reaction by lowering its activation energy.
However, motivated partly by the recognition that 'Nature has designed'
enzymes which are more efficient than the catalysts discovered by Man,
and partly by industry's demands for more efficient catalysts (so as to
reduce energy costs), researchers have begun to unravel the details of
catalysis. Most unfortunately, even a superficial understanding of this
research requires a fairly advanced knowledge of atomic structure and
chemical bonding; on the other hand, even a 'bird's-eye-view' of one
reaction can provide a useful point of reference ...
Aqueous dibromine is but one of several substances known to catalyze
the decomposition of aqueous hydrogen peroxide; i.e.,
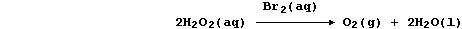
The experimental evidence is consistent with the interpretation that
this reaction occurs essentially in two steps. In the first, dibromine
oxidizes hydrogen peroxide to aqueous hydrogen ions and dioxygen; i.e.,
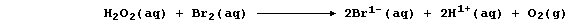
Then, in the second, the bromide ions are oxidized back to dibromine by
more hydrogen peroxide; i.e.,
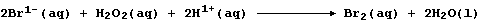
Thus, dibromine reacts in the first step and is then regenerated in the
second; so, the substance is not used up in the reaction.
Each catalyst increases the rate of a reaction without being chemically
changed at the end of a reaction. Accordingly, each reaction involving
a true catalyst can always be represented by a closed loop; the various
catalytic species form the main body of the loop, and the reactants and
products enter and leave the loop at various places.
Shown below is a closed loop for the dibromine-catalyzed decomposition
of aqueous hydrogen peroxide.
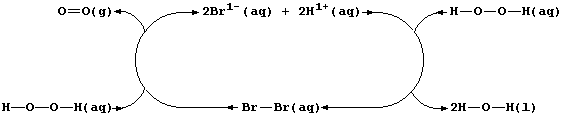

1. Nitrogen-fixation is the term used to describe the conversion of
molecular dinitrogen into compounds of nitrogen.
(a) Industrial fixation involves the reduction of dinitrogen to ammonia
using an iron catalyst, high temperatures, and high pressures; e.g.,
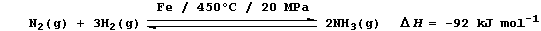
Complete and label this energy level diagram for the above reaction.
By reference to the Periodic Table, name two elements which might show
similar catalytic activity to iron. ___________________________________
[2]
One mole of any gas occupies a volume of 24000 cm³ at room temperature
(25°C) and pressure (0.1 MPa). Calculate the density of ammonia at
r.t.p. ________________________________________________________________
_______________________________________________________________________
[2]
(b) Biological fixation involves the Mo-containing enzyme nitrogenase,
under ambient conditions, in an ostensibly similar reduction; one of
the important reactions can be loosely summarized by this equation: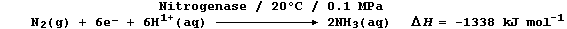
Suggest and explain the reason why this reaction is considerably more
exothermic than that which occurs in industrial fixation. _____________
_______________________________________________________________________
_______________________________________________________________________
[2]
(c) Atmospheric fixation results in the formation of various nitrogen
oxides. Name two sources of energy involved in this type of nitrogen-
fixation. _____________________________________________________________
[2]
2. Carbon-fixation is the term used to describe the incorporation of
carbon dioxide into organic compounds; as occurs, for example, during
the biological process of photosynthesis: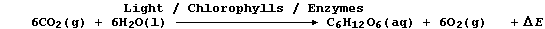
The kingdom Monera consists of three subkingdoms: Eubacteria (formerly
Bacteria; e.g., Bacillus anthracis, Clostridium botulinum, Escherichia
coli, Treponema pallidum, and Vibrio cholerae); Cyanobacteria (formerly
Blue-green Algae; e.g., Nostoc commune); and Archaebacteria (e.g.,
Methanococcus jannaschii). The most nutritionally independent organisms
in the biosphere are species of Cyanobacteria which can carry out both
nitrogen-fixation and carbon-fixation (via photosynthesis). State what
these organisms require for survival, apart from a suitable temperature
and sunlight. _________________________________________________________
[4]
SELECTED PRINCIPLES: EQUILIBRIA (1)
A chemical reaction can be executed in three types of system. First,
an isolated system, which is one that does not allow either energy
or matter to be transferred (to its surroundings); an example, albeit
imperfect, is a thermos flask. Second, a closed system, which is one
that allows energy but not matter to be transferred; e.g., a sealed
conical flask. And third, an open system, which is one that allows both
energy and matter to be transferred; e.g., an open conical flask.
In principle, each chemical reaction is reversible; i.e., it can occur
in both directions. Consider this abstract reaction in a closed system:
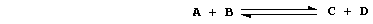
When the two reactants A and B are mixed, only the forward reaction is
possible. As it continues, the numbers of A and B molecules decrease:
so, the forward reaction slows down. At the same time, the numbers of
C and D molecules increase: so, the reverse reaction speeds up. And,
at a constant temperature and pressure, the system eventually reaches a
state of dynamic equilibrium; i.e., the forward and reverse reactions
occur at equal rates, and the net concentrations of A, B, C, and D
molecules remain constant.
The term 'position of equilibrium' refers to one particular state of
a closed system; and one state, or position, differs from another in
the concentrations of substances present in the equilibrium mixture.
Concentration, pressure, and temperature are three of the independent
variables known to affect the position of equilibrium; the qualitative
effect of changing such variables is summarized by Le Chatelier's
Principle: 'When a closed system at dynamic equilibrium is subjected to
change in an independent variable, the position of equilibrium shifts
so as to oppose the effect of the imposed change.'.
Thus, at dynamic equilibrium in a closed system,
... an increase in the concentrations of products shifts the position
of equilibrium to the left.
... an increase in the concentrations of reactants shifts the position
of equilibrium to the right.
... an increase in pressure increases the yield when there are fewer
moles of gas on the product side of the equation.
... an increase in pressure decreases the yield when there are more
moles of gas on the product side of the equation.
... a change in pressure has no effect on the yield when there are the
same number of moles of gas on each side of the equation.
... an increase in temperature increases the yield when the reaction is
endothermic (+ DH)
... an increase in temperature decreases the yield when the reaction is
exothermic (- DH).
1. Shown below is a flow diagram for the manufacture of ammonia by the
Haber process; and the following equation summarizes its synthesis by
the set of reaction conditions most commonly used in industry.
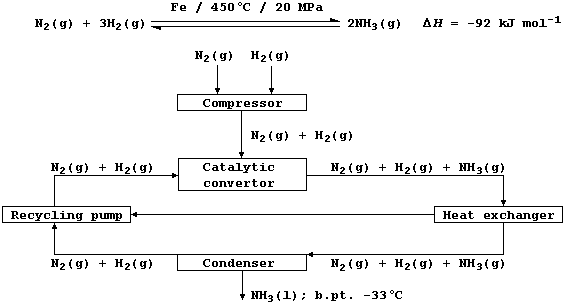
(a) Dinitrogen (b.pt. -196°C) is obtained by fractional distillation of
liquid air. State two methods of obtaining dihydrogen (b.pt. -253°C).
_______________________________________________________________________
[2]
(b) Using the conditions summarized in the above equation, the yield of
ammonia is about 28% at equilibrium. Complete the following sentences,
so as to summarize the effects of three independent variables, on the
yield of ammonia and the rate of reaction, in this process.
The high pressure used __________ the yield, because the position of
equilibrium moves to oppose the increase in pressure, and __________
the rate, because there are more collisions between the particles.
The high temperature used __________ the yield, because the position of
equilibrium moves to oppose the increase in temperature of this
exothermic reaction, but __________ the rate, because more particles
have the required activation energy for successful collisions.
The catalyst used has __________ on the yield, because it affects the
forward and reverse reactions equally, but __________ the rate, because
it lowers the activation energy and so more particles have the required
energy for successful collisions.
[6]
2. A research chemist prepared a sample of labelled ammonia, using the
isotope nitrogen-15. This sample was heated with iron, at 450°C under
high pressure (20 MPa) in a closed system, until the composition of the
mixture was constant. For the reaction studied, construct the symbol
equation complete with heat energy change. ____________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
3. Ammonia, a colourless gas, is very soluble in water (approximately
68000 cm³ dissolves in 100 cm³ of water at 25°C and 100 kPa; cf. with
1.5 and 3.0 cm³ for dinitrogen and dihydrogen, respectively). Ammonia's
exceptionally high solubility in water is attributable to 'hydrogen
bonding'; i.e., the weak electrostatic attraction between the nucleus
of a bonded hydrogen atom and a lone-pair of electrons of a bonded
non-hydrogen atom (typically, nitrogen, oxygen, or fluorine). Calculate
the concentration, in mol dm-³, of a saturated solution of ammonia. ___
_______________________________________________________________________
[2]
SELECTED PRINCIPLES: EQUILIBRIA (2)
the maximum yield in any given industrial process in the minimum time.
Not surprisingly, therefore, the variables which affect the yield and
the rate of a reversible reaction are important considerations in plant
design; and, for example, certainly would have been in the optimization
of one industrial method of manufacturing ethanoic acid, which involves
the catalyzed, gas-phase reaction between methanol and carbon monoxide.
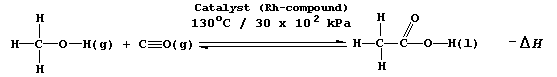
[Scene. A tea-party in the plush boardroom of Hydragyrum Chapelier
Vinaigrette (a small company which, most curiously, manufactures hats
and ethanoic acid); the chairman's name is Monsieur Oliver Scrooge.]
O. Scrooge: We cannot rest on our laurels of the past. So, I want
more, and I want it faster! [The board members ignore
this party pooper.] Otherwise, ... each of you will be
collecting your Christmas present from the Government.
[With this veiled threat, Les Femmes are all attention.]
La Vitesse: Well, ... a catalyst would increase the rate. However,
it is a compound of a precious metal: ... so there would
be a major capital cost. Nevertheless, we can re-use
the catalyst ... providing, of course, we minimize the
introduction of inhibitors into the reaction vessels.
O. Scrooge: Will it increase the yield? [His tone is hopeful.]
La Vitesse: Certainly not! [S. looks glum.] No catalyst changes
the position of equilibrium. On the other hand, without
a catalyst, we would definitely need to use much higher
temperatures: ... so, indirectly, it would reduce costs.
La Chaleur: True, though a high temperature would increase the rate.
O. Scrooge: Yes, ... but will a high temperature increase the yield?
La Chaleur: Certainly not! Do pay attention! The reaction is
exothermic. [S. looks chastened and even more glum.]
La Pression: A high pressure would also increase the rate ... though,
again, with increased operating costs.
O. Scrooge: Yes, yes, ... [His tone is weary.] ... but will a high
pressure increase the yield?
La Pression: Certainly! [S. perks up.] To echo La Chaleur, do pay
attention! Note that there are fewer moles of gas on
the product side of the equation ... well, none in fact.
O. Scrooge: How absolutely splendid! I suggest that we should be
miserly with the catalyst, use a lowish temperature, and
boost the pressure massively. [His tone is bullish.]
La Securité: No, ... not necessarily. I must urge caution. Reaction
vessels strong enough to withstand very high pressures
are exceedingly expensive. The safety of our workers
and the general public is paramount!
O. Scrooge: Oh dear, are all females of the species this sensible?
[His tone is ambiguous, perhaps even patronizing.]
La Securité: Certainly: ... well, most of the time. However, we will
suspend judgement on your future, ... [Les Femmes glance
pointedly at the sharp stiletto heels on their shoes.]
... until after we have received our Christmas bonuses.
O. Scrooge: [He picks up a telephone.] Bob, is that you? Please
come back ... |
1. One (no longer used) method of obtaining hydrogen chloride involved
the copper(II)-catalyzed reduction of chlorine;

(a) Complete the Table below using these bond energies (in kJ mol-¹):
463 (O-H), 243 (Cl-Cl), 432 (H-Cl), and 497 (O=O).
Bonds broken |
Energy absorbed
/ kJ mol-¹ |
Bonds formed |
Energy released
/ kJ mol-¹ |
|
|
|
|
|
|
|
|
Total = |
Total = |
[3]
Calculate the heat energy change (DH) for the above reaction. _________
_______________________________________________________________________
[2]
(b) Explain the purpose of the broken brick. __________________________
_______________________________________________________________________
[2]
(c) Complete and label this energy level diagram for the reaction.
Energy ___
___
_________________
_________________
___________________ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
___________________
_________________________________________________
Path of reaction
[5]
(d) Explain the effects, on the rate of this reaction and the yield of
hydrogen chloride, of using:
A high pressure _______________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
A high temperature ____________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[4]
2. In industry, hydrogen chloride is usually obtained as a co-product
of the manufacture of chlorinated hydrocarbons; e.g.,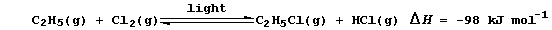
(a) Suggest the rôle of light in this (substitution) reaction. ________
_______________________________________________________________________
[1]
(b) State briefly why the use of a high pressure in this reaction would
not increase the yield of products. ___________________________________
_______________________________________________________________________
[1]
(c) Suggest one advantage that this industrial method has over that
described in 1. _______________________________________________________
[1]
SELECTED PRINCIPLES: ACIDS & BASES (1)
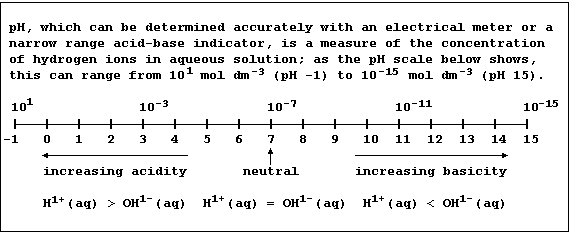
Certain definitions, which are intentionally narrow, will be useful in
this introduction to acid-base chemistry. First, acids are substances
which produce hydrogen ions when dissolved in water. Second, bases are
substances which accept hydrogen ions (e.g., the hydroxides, oxides,
sulfides, hydrogencarbonates, and carbonates of metals); those which
are soluble in water are known as alkalis. Third, salts are ionic
compounds which contain ions other than hydrogen, hydroxide, or oxide.
And finally, neutralization is the reaction between an acid and a base.
When hydrogen chloride is dissolved in an organic solvent, the solution
formed does not conduct an electric current - indicating the absence of
free-moving ions. By contrast, when this gas is dissolved in water,
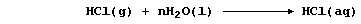
the aqueous solution of hydrochloric acid formed conducts an electric
current strongly, because this 'strong' acid is completely dissociated;
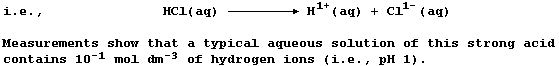
When sodium hydroxide is dissolved in water,
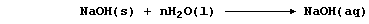
the aqueous solution of sodium hydroxide formed conducts an electric
current strongly, because this 'strong' base is completely dissociated;
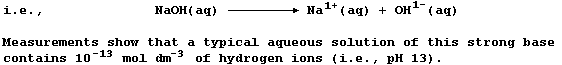
The symbol equation for the neutralization reaction between aqueous
solutions of nitric acid and sodium hydroxide is:
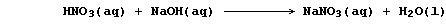
Because the reactants are completely dissociated, a better perspective
of this reaction is gained by constructing an ionic equation;
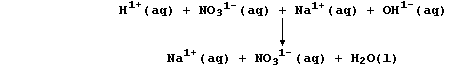
Those ions that are not involved in the overall reaction, here aqueous
sodium and nitrate, are referred to as 'spectator' ions; and, so as to
focus on the change that actually occurs in this neutralization, a net
ionic equation can be written; i.e.,
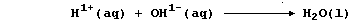
The measured heat energy change (DH) for this (exothermic) reaction is
-56 kJ mol-¹.
1. The symbol equation for the neutralization reaction between aqueous
solutions of sulfuric acid and potassium hydroxide is:
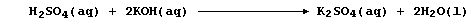
(a) State the spectator ions in this reaction. ________________________
[2]
(b) Construct the net ionic equation for this reaction. _______________
_______________________________________________________________________
[2]
2. In humans, both pancreatic juice and bile are rich in hydrogen-
carbonate ions; these neutralize the acidity of the chyme flowing from
the stomach, as well as provide the slightly alkaline pH required for
optimal activity of the pancreatic enzymes. The symbol equation for the
neutralization reaction, which occurs in the duodenum, is: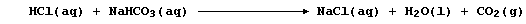
(a) Construct the ionic equation for this reaction. ___________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) Describe one other rôle of bile in digestion. _____________________
_______________________________________________________________________
[2]
3. Acidic gases are released into the atmosphere when fossil fuels are
burnt. The condensation of atmospheric water vapour containing these
gases, and others formed in the atmosphere, leads to the precipitation
of 'acid rain' - which is a mixture of several acids. Depending on the
weather conditions and location, this rain or snow can have a pH of
between 2.2 and 5.5: by contrast, unpolluted rainwater has a pH of 5.6.
(a) State the percentage increase in hydrogen ions of rainwater with a
pH of 2.6 compared to that with a pH of 5.6. __________________________
[1]
(b) Hydrated metal ions usually enter living cells by diffusion across
semi-permeable membranes. In unpolluted environments, the concentration
of most toxic metal ions is very low because these ions are 'locked-up'
in insoluble compounds [e.g., aluminium oxide, cadmium(II) carbonate,
lead(II) sulfide, and mercury(II) sulfide]: but, such compounds react
with the components of acid deposition - as a result, the concentration
of toxic hydrated ions increases markedly in polluted environments.
Contruct the symbol equation for the neutralization reaction between:
Dilute nitric acid and aluminium oxide ________________________________
_______________________________________________________________________
Dilute nitric acid and lead(II) sulfide _______________________________
_______________________________________________________________________
[4]
(c) Acidified soils are often treated with either quicklime, CaO(s), or
slaked lime, whereas acidified lakes are usually treated with powdered
limestone. Construct a symbol equation for the neutralization reaction
between:
Dilute sulfuric acid and quicklime ____________________________________
_______________________________________________________________________
Dilute sulfuric acid and limestone ____________________________________
_______________________________________________________________________
[4]
(d) Saprotrophic organisms are vitally important in the recycling of
chemical energy and nutrient ions within the biosphere. Suggest and
explain one reason why the growth and reproductive rates of saprotrophs
usually decrease in acidic environments. ______________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
SELECTED PRINCIPLES: ACIDS & BASES (2)
When ethanoic acid is dissolved in an organic solvent, the solution
formed does not conduct an electric current - indicating the absence of
free-moving ions. By contrast, when this liquid is dissolved in water,
the aqueous solution of ethanoic acid formed conducts an electric
current weakly, because this 'weak' acid is partially dissociated;
i.e., the position of the following equilibrium lies far to the left:
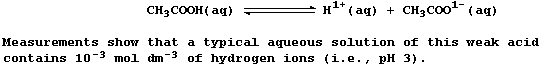
Water is often considered to be a purely covalent compound. However,
even after extensive purification by repeated distillation, the purest
sample of water still conducts an electric current very weakly. The
explanation for this observation is that water undergoes very slight
dissociation (or ionization); the position of the following equilibrium
lies almost completely to the left:

When ammonia gas is dissolved in water,
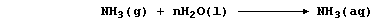
the aqueous solution of ammonia formed conducts an electric current
weakly, because this 'weak' base is partially dissociated; i.e., the
position of the following equilibrium lies far to the left:
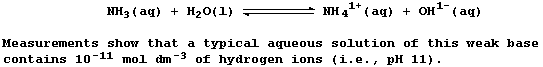
The descriptive terms 'strong' and 'weak', used in reference to acids
and bases, are simply a qualitative measure of the extent of ionization
or dissociation in water; they do not refer to the concentration of
substance: so, oxymoronic phrases such as 'concentrated weak acid' and
'dilute strong base' are perfectly acceptable - as this Table reveals.
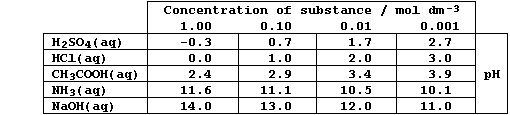

1. Collisions between free-moving ions in aqueous solutions usually
occur so frequently that most ionic reactions, including neutralization
and precipitation, are immeasurably fast. The 'instantaneous' character
of neutralization allows various hypotheses to be investigated; e.g.,
'The speed (S) at which aqueous ethanoic acid 'ice-cubes' dissolve in
aqueous sodium hydroxide is in direct proportion to their surface area
(A); i.e., S = k × A'.
(a) Construct the symbol equation for the neutralization reaction
between aqueous solutions of ethanoic acid and sodium hydroxide. ______
_______________________________________________________________________
[2]
State the spectator ions in this reaction. ____________________________
[2]
(b) Suggest three independent variables which must be measured and held
constant in order to isolate the independent variable of surface area.
_______________________________________________________________________
_______________________________________________________________________
[3]
(c) What type of substance would be used to determine that an ethanoic
acid 'ice-cube' had completely dissolved in aqueous sodium hydroxide?
_______________________________________________________________________
[1]
(d) Calculate the total surface of one 'ice-cube' if the surface area
of one face is 4 cm². _________________________________________________
[1]
What physical property of ice results in its effective surface area in
contact with an aqueous solution being less than that calculated? _____
_______________________________________________________________________
[1]
(e) Calculate the volume of one 'ice-cube' if the surface area of one
face is 4 cm². ________________________________________________________
[1]
If the concentration of the aqueous ethanoic acid used to make the
'ice-cubes' is 0.125 mol dm-³, calculate the number of moles of acid in
each 'ice-cube'. ______________________________________________________
[2]
2. Despite the abundance of atmospheric dinitrogen, the availability
of usable nitrogen is normally the most important factor limiting plant
growth: so, to sustain or increase crop yields, farmers use artificial
nitrogenous fertilizers. The manufacture of these compounds is typified
by that of ammonium hydrogenphosphate; this involves the neutralization
reaction between aqueous solutions of phosphoric acid and ammonia,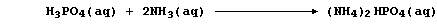
followed by the controlled evaporation of excess water,
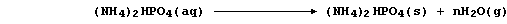
(a) Suggest one reason why the evaporation of an aqueous solution of an
ammonium salt must be carefully controlled. ___________________________
_______________________________________________________________________
[1]
(b) Complete this description of one method of preparing crystals of
ammonium hydrogenphosphate. "Wearing safety glasses, transfer 20.0 cm³
of aqueous ammonia (1.00 mol dm-³) to an insulated conical flask, using
a pipette and safety-______; then measure the starting temperature. Add
rapidly _________ of aqueous phosphoric acid (1.00 mol dm-³), using a
a burette; then measure the final temperature of the reaction mixture.
Evaporate this solution, using a ___________________, until crystals
appear; then leave the crystalline mixture to dry at room temperature."
[3]
(c) Suggest one reason why autotrophic organisms require a source of
phosphate. ____________________________________________________________
[1]
SELECTED PRINCIPLES: REDOX REACTIONS (1)
The word redox is a contraction of 'reduction-oxidation'. Historically,
oxidation was used to describe the addition of oxygen to a reactant;
e.g., in this first reaction, magnesium has been oxidized by dioxygen
(which is acting as the oxidizing agent or oxidant):
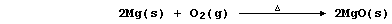
Conversely, reduction described the removal of oxygen from a reactant;
e.g., in this second reaction, copper(II) oxide has been reduced by
dihydrogen (which is acting as the reducing agent or reductant):
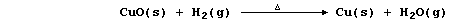
Close inspection of the above reactions reveals two important points.
First, when one reactant is reduced, the other is oxidized (... redox).
And second, when a particle is oxidized it loses electrons, and when a
particle is reduced it gains electrons; e.g.,

This second point focuses on electron transfer in redox reactions, and
this is the basis for one modern definition of oxidation and reduction:
namely, oxidation is the loss of electrons (Olé!), and reduction is the
gain of electrons.
Most recently, another definition of oxidation and reduction has found
widespread favour: namely, one based on changes in oxidation numbers
(often called oxidation states). Because a comprehensive presentation
of oxidation numbers is beyond the scope of this text, a brutal summary
will have to suffice: oxidation is an increase in oxidation number, and
reduction is a decrease in oxidation number ... as illustrated below.
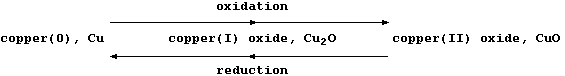
The oxidation number or state, (in Roman numerals), is
placed immediately after the name of the element concerned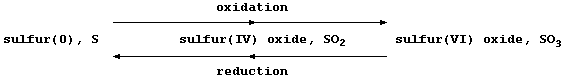
I = 1 II = 2 III = 3 IV = 4 V = 5 VI = 6 VII = 7

1. Oxidizing agents are substances which cause oxidation; examples of
these reagents used in laboratories include potassium dichromate(VI),
concentrated tetraoxosulfuric(VI) acid, and potassium manganate(VII).
(a) Potassium dichromate(VI), dissolved in aqueous sulfuric acid, is a
convenient reagent for the oxidation of alcohols to their corresponding
carboxylic acids; e.g., propan-1-ol is oxidized to propanoic acid:
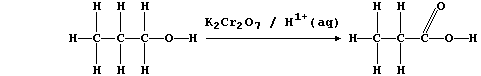
In this reaction, the orange dichromate(VI) ions are reduced to green
chromium(III) ions; a simplified redox half equation is as follows:
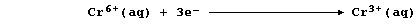
Recalling that the number of electrons equals the number of protons in
a neutral atom of an element, state the number of electrons there are
in each of these particles:
24Cr __________ 24Cr3+ __________ 24Cr6+ __________ 18Ar ___________
[2]
(b) Concentrated tetraoxosulfuric(VI) acid, better known as sulfuric
acid, oxidizes hydrogen bromide as follows: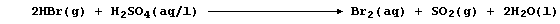
State the change in oxidation number of sulfur. _______________________
[1]
Suggest two observations which should be made during this reaction. ___
_______________________________________________________________________
[2]
(c) Potassium manganate(VII), dissolved in aqueous potassium hydroxide,
is a reagent sometimes used to test for the presence of a C=C double
bond; e.g., cis-but-2-ene is oxidized to butane-2,3-diol: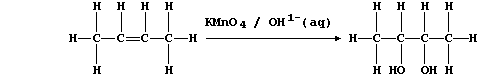
In this reaction, the purple manganate(VII) ions are reduced to brown
manganese(IV) ions; a simplified redox half equation is as follows:
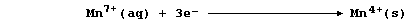
State the number of electrons there are in each of these particles:
25Mn __________ 25Mn4+ __________ 25Mn7+ __________ 18Ar ___________
[2]
2. Reducing agents are substances which cause reduction; examples of
these reagents used in laboratories include magnesium(s), methane(g),
and carbon-graphite(s). Construct a symbol equation for the reduction
of lead(IV) oxide to lead(0) with each of these named reducing agents.
_______________________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
[6]
3. Lead(II) ions are very toxic to most living organisms because they
inhibit the 'active sites' of a wide variety of enzymes; in particular,
those which are catalysts in the biosynthesis of compounds involved,
directly or indirectly, in the redox processes of either photosynthesis
or cellular respiration or both (e.g., chlorophylls, cytochromes, and
haemoglobin). Name the condition caused by a shortage of haemoglobin,
the dioxygen-carrying pigment in mammalian red blood cells. ___________
[1]
SELECTED PRINCIPLES: REDOX REACTIONS (2)
Reducing agents are substances which cause reduction, and oxidizing
agents are substances which cause oxidation. This uninspiring sentence,
though correct, obscures the fact that the terms 'reducing agent' and
'oxidizing agent' are relative, as the two following examples of redox
reactions should illuminate.
First, magnesium and dihydrogen are both termed reducing agents: but
magnesium is the stronger one. Thus, in the following redox reaction,
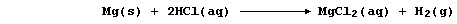
electrons are transferred from magnesium atoms to hydrogen ions because
magnesium is a stronger reductant (i.e., it is more easily oxidized);
the redox half equations are:
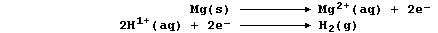
The aqueous chloride ions are spectators, so the net ionic equation is:
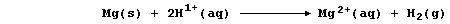
And second, both bromine and iodine are termed oxidizing agents: but
bromine is the stronger one. Thus, in the following redox reaction,
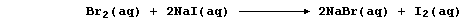
electrons are transferred from iodide ions to bromine atoms because
bromine is a stronger oxidant (i.e., it is more easily reduced); the
redox half equations are:
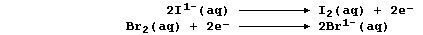
The aqueous sodium ions are spectators, so the net ionic equation is:
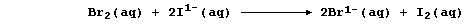
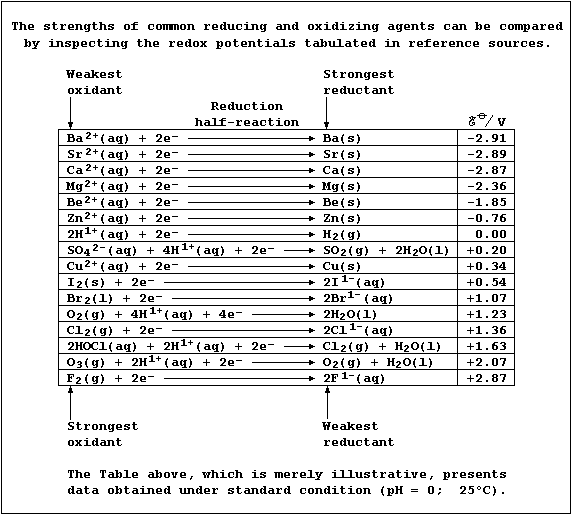
1. The elements in Group 17 are collectively known as the halogens.
All isotopes of astatine are radioactive; its longest-lived isotope is
astatine-210, which has half-life of 8.3 hours: therefore, it is both
difficult and expensive to study astatine in a laboratory.
(a) Complete this first Table, by predicting properties of diastatine.
Property |
F2 |
Cl2 |
Br2 |
I2 |
As2 |
Melting point / °C |
-223 |
-102 |
-7 |
114 |
|
Boiling point / °C |
-188 |
-35 |
59 |
183 |
|
Appearance at r.t.p.
(25°C; 100 kPa) |
pale
yellow
gas |
yellow-
green
gas |
red-
brown
liquid |
dark
grey
solid |
|
Bond energy / kJ mol-¹ |
151 |
243 |
193 |
151 |
|
[4]
(b) Complete this second Table, to show the radioactive decay of At-210.
Time / hours |
0.0 |
8.3 |
16.6 |
|
|
|
Number of half-lives elapsed |
0 |
1 |
2 |
|
|
|
Mass of 210As / 10-9 g |
96 |
48 |
24 |
|
|
|
[3]
2. Chlorine gas dissolves in water to give so-called 'chlorine water',
a rather loose term used to indicate the following equilibrium mixture:
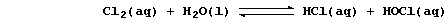
The hydrochloric acid is completely dissociated, being a strong acid,
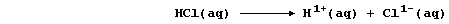
and the chloric(I) acid is partially dissociated, being a weak acid,
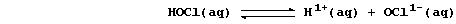
Aqueous hydrogen and chlorate(I) ions both diffuse into cells, across
semi-permeable membranes, where hydrogen ions can denature enzymes
and chlorate(I) ions act as biocides by oxidizing essential biological
molecules. Name two types of molecules, which are involved in growth,
reproduction, or respiration, which might be oxidized by chlorate(I)
ions. _________________________________________________________________
[2]
3. Shown below is a flow diagram for the extraction of bromine from
sea water, where it occurs as bromide ions (their concentration is low;
about 0.068 g dm-³).
(a) Construct the net ionic equation for the reactions at Stages B and
E, which involves dichlorine acting as the oxidizing agent. ___________
_______________________________________________________________________
[1]
(b) Construct the symbol (or ionic) equation for the reaction at Stage
D, which involves sulfur(IV) oxide acting as the reducing agent. ______
_______________________________________________________________________
[2]
(c) Suggest one method of separating dibromine from the aqueous mixture
at Stage F. ___________________________________________________________
[1]
SELECTED PRINCIPLES: ELECTROLYSIS (1)
Electrolytes are substances which contain free-moving ions; examples
include molten salts, and aqueous solutions of acids, bases, and salts.
And electrolysis is the decomposition of an electrolyte by an electric
current, as a result of redox reactions which occur at electrodes.
The synthesis of aqueous zinc bromide is an exergonic process; i.e.,
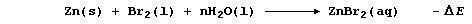
Not surprisingly, therefore, but with a delightful feeling of logic,
the decomposition of aqueous zinc bromide by an electric current is an
endergonic process; i.e.,
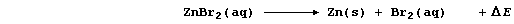
The diagram shows an external circuit connected to an electrolytic cell
consisting of two carbon-graphite electrodes immersed in an aqueous
solution of zinc bromide; the ions present are Zn2+(aq), Br1-(aq), and,
as a result of the ionization of water, H1+(aq) and OH1-(aq).
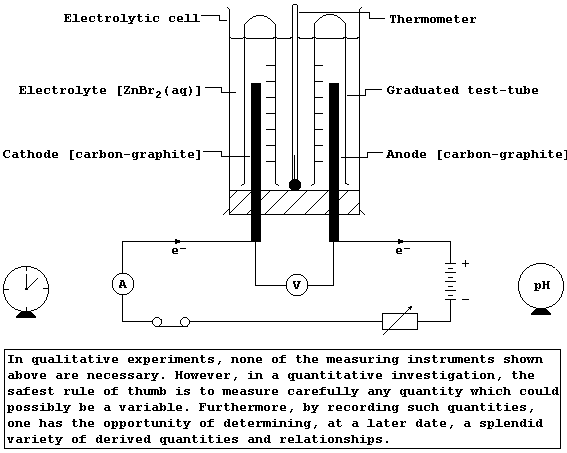
When a small, direct electric current is passed through this (initially
colourless) electrolyte, an orange-brown solution is formed around the
electrode connected to the positive side of the battery, and a grey
solid is deposited on the electrode connected to its negative side;
these observations are interpreted as follows.
The negatively charged bromide anions migrate to the positively charged
anode, where they are discharged by losing electrons; i.e.,
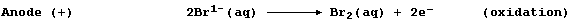
These electrons are forced round the external circuit to the cathode,
by the electrical energy transduced from the battery's chemical energy.
The positively charged zinc cations migrate to the negatively charged
cathode, where they are discharged by gaining electrons; i.e.,
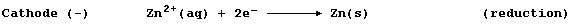
1. Explain why solid ionic compounds are non-electrolytes. ___________
_______________________________________________________________________
_______________________________________________________________________
[2]
2. The synthesis of (liquid) water can be summarized by this equation: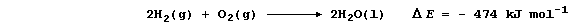
Construct a similar equation for the decomposition of (liquid) water.
_______________________________________________________________________
[1]
3. In principle, the ease of discharge of ions from aqueous solutions
should correspond to their redox potentials: in practice, however,
other variables are known to be important. Because the details of these
complications are beyond the scope of this text, two predictive rules
of thumb are presented here (when 'inert' electrodes are used): first,
the anodic product is usually dioxygen, unless the electrolyte contains
halide ions; and second, the cathodic product is always a metal or
dihydrogen, and is usually this gas if the metal is more reactive than
zinc. The Table below summarizes the reactions of various electrolytes
at carbon graphite electrodes. Complete this Table, by inserting the
correct substance from this list: CuCl2(aq), HCl(aq), H2O(l), HNO3(aq),
H2SO4(aq), MgCl2(aq), MgCl2(l), MgCl2(s), MgSO4(aq), and ZnI2(aq).
Substance
|
Reaction(s) at anode
|
Reaction(s) at cathode
|
|
None
|
None
|
|
2Cl1-(aq) ® Cl2(g) + 2e-
|
Mg2+(aq) + 2e- ® Mg(l)
|
|
2Cl1-(aq) ® Cl2(g/aq) + 2e-
|
2H1+(aq) + 2e- ® H2(g)
|
|
2I1-(aq) ® I2(aq) + 2e-
|
Zn2+(aq) + 2e- ® Zn(s)
|
|
2Cl1-(aq) ® Cl2(aq) + 2e-
|
Cu2+(aq) + 2e- ® Cu(s)
|
|
None
|
None
|
|
2Cl1-(aq) ® Cl2(g/aq) + 2e-
|
2H1+(aq) + 2e- ® H2(g)
|
|
4OH1-(aq) ® O2(g) + 2H2O(l) + 4e-
|
4H1+(aq) + 4e- ® 2H2(g)
|
|
4OH1-(aq) ® O2(g) + 2H2O(l) + 4e-
|
4H1+(aq) + 4e- ® 2H2(g)
|
|
4OH1-(aq) ® O2(g) + 2H2O(l) + 4e-
|
4H1+(aq) + 4e- ® 2H2(g)
|
[5]
Pure water conducts an electric current, albeit very weakly, because it
undergoes very slight ionization: nevertheless, there are too few ions
for significant electrolysis to occur. However, as the Table shows, the
electrolysis of water does occur when an ionic substance is present.
Suggest two more substances, one a soluble salt and the other an acid,
which would have a similar effect. ____________________________________
[2]
SELECTED PRINCIPLES: ELECTROLYSIS (2)
Practical applications of electrolysis include the extraction of metals
(e.g., sodium), the manufacture of compounds (e.g., sodium hydroxide),
and both the electroplating and purification of metals (e.g., copper).
These applications make use, indirectly, of two laws of electrolysis
determined by Michael Faraday (1791 - 1867). His First Law states 'that
the mass of a substance produced at an electrode during electrolysis is
proportional to the quantity of electricity passed'. And his Second Law
states 'that the quantity of electricity required to produce one mole
of a substance from its ions is proportional to the charge on those
ions'. Together, these two laws are summarized by two nifty equations,
Q = I × t and Q = n × z × F
where: Q, measured in coulombs (C), is the quantity of electricity;
I, measured in amps (A), is the current; t, measured in seconds (s),
is the time; n is the number of moles of substance produced at the
electrode; z is the charge on the ion; and, F is a constant, with a
value of 96500 C mol-¹.
This diagram shows a circuit, which includes three electrolytic cells
connected in series, through which a current of 0.75 A was passed for
45 minutes; so, the quantity of electricity (Q) which passed through
this circuit was: Q = I × t = 0.75 × 45 × 60 = 2025 C. And the results
of this experiment are summarized in the Table below; its careful study
should prove rewarding.

[Note that each anode in this experiment is an 'active' electrode; so,
overall, there is a net transfer of metal atoms from anode to cathode.]
An(ode) ... There once was a damsel called Goldilocks, whose hobby was
counting odd sox. She sat in her box, and said pox on you fox, because
she always got flummoxed by red and by ox. So along came three bears,
who rushed up the stairs, to sort out this mess for a lass in distress.
And out of the blue came some bees, if you please, to see if they could
help her too. There's no need to fuss, if you want the A plus, because
an ode is so easy to suss. Simply feign you're in Spain, we know it's
insane, and sing in the rain ... Olé! Honey! |
1. The subject of the equation Q = n × z × F is the quantity
of electricity (Q). Rearrange this equation so that its subject is the
number of moles (n) of substance produced. ____________________________
The subject of the equation Q = I × t is also Q. Rearrange this
equation so that its subject is the current (I). ______________________
[2]
2. Sea water is a rich source of magnesium ions (their concentration
is about 1.33 g dm-³), and magnesium chloride is obtained from this
resource by concentration followed by fractional crystallization. One
method of extracting magnesium metal is by the electrolysis of molten
magnesium chloride at about 750°C; i.e.,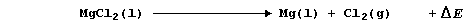
A typical electrolytic cell used in industry operates at a current (I)
of 200 kA. Determine the mass (m) of magnesium metal that would be
obtained in 12 hours - as follows.
Calculate the quantity of electricity used in 12 hours. _______________
_______________________________________________________________________
Calculate the number of moles of magnesium produced at the cathode. ___
_______________________________________________________________________
And finally, calculate the mass of magnesium obtained. ________________
_______________________________________________________________________
[5]
State the number of moles of dichlorine that would be evolved at the
anode during the same 12 hour period. _________________________________
[1]
3. Shown below are electron-structure diagrams for atomic chlorine and
molecular chlorine (or dichlorine).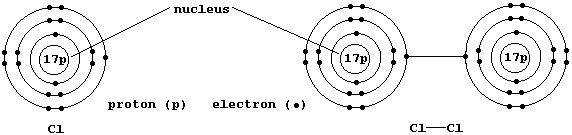
State briefly why a chlorine atom is considered to be a free-radical.
_______________________________________________________________________
[1]
4. In an experiment executed at room temperature and pressure, using
a cell equipped with carbon-graphite electrodes and gas burettes, an
aqueous solution of hydrochloric acid was electrolyzed; i.e.,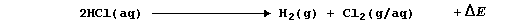
A current (I) of 0.75 A was passed through this electrolytic cell for a
period of time; 25.2 cm³ of dihydrogen was collected over the cathode
and 23.7 cm³ of dichlorine was collected over the anode. Recalling that
the volume of one mole of any gas at r.t.p. is 24000 cm³, determine the
time (t) taken to obtain this volume of dihydrogen - as follows.
Calculate the number of moles of molecular hydrogen evolved at the
cathode. ______________________________________________________________
State the number of moles of atomic hydrogen, H(g), produced at the
cathode _______________________________________________________________
Calculate the quantity of electricity required to evolve this number of
moles of hydrogen atoms. ______________________________________________
And finally, calculate the time taken for this quantity of electricity
to be used. ___________________________________________________________
[6]
Suggest one reason why the volume of gaseous dichlorine collected was
significantly less than that of dihydrogen. ___________________________
_______________________________________________________________________
[1]
SELECTED PRINCIPLES: ELECTROCHEMICAL CELLS
An electrochemical cell transduces chemical energy to electrical energy
when it is part of a complete electrical circuit.
An exothermic reaction occurs when a piece of zinc metal is placed in
aqueous copper(II) sulfate; i.e.,
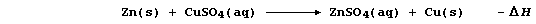
In this redox reaction, electrons are transferred from zinc atoms to
copper(II) ions because zinc is a better reducing agent (i.e., it is
more easily oxidized); the redox half equations are:
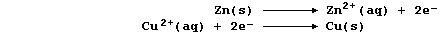
In such an experiment, the energy transduced is released as heat energy
because the electrons are transferred directly from the reductant to
the oxidant in solution. However, when the reductant is physically
separated from the oxidant, then the electrons can be transferred via
an external circuit; and so, as a reaction proceeds, chemical energy
will be transduced to electrical energy.
An electrochemical cell is constructed by physically separating the
reductant from the oxidant; a typical example, connected to an ammeter
and a filament lamp, is shown in the diagram below.
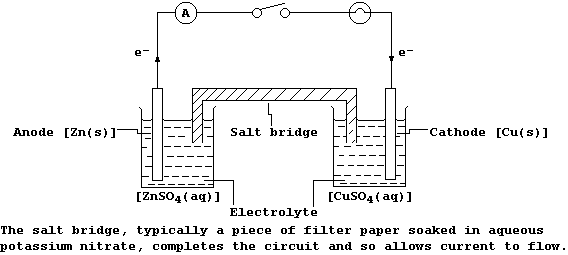
When the switch is closed, the ammeter registers a reading and the lamp
lights; these observations are interpreted as follows.
At the anode, zinc atoms lose two electrons, and so the mass of zinc
decreases and the concentration of aqueous zinc ions increases; i.e.,
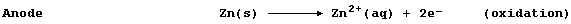
These electrons flow through the wire of the external circuit - where
their electrical energy is transduced to heat and light energy of the
filament lamp - to the copper electrode. At this cathode, copper(II)
ions gain two electrons, and so the concentration of aqueous copper(II)
ions decreases and the mass of copper increases; i.e.,
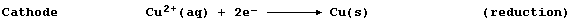
Finally, if both the ammeter and the lamp are replaced by a voltmeter,
then the above cell will register its maximum potential difference (or
voltage); this value is +1.10 V, when each solution has a concentration
of 1.00 mol dm-³ and the temperature is 25°C (298 K).

1. The energy change (DE) which occurs when a cell transduces chemical
to electrical energy can be calculated using this equation,
DE = - n × F × V
where: n is the number of electrons transferred; F is a constant, with
a value of 96500 C mol-¹; and, V is the potential difference (p.d.).
Complete the Table below, using this equation to calculate the energy
change for each cell; the number of electrons transferred, n, is two.
Anode |
Cathode |
Cell p.d. / V |
Energy change (DE) / kJ mol-¹ |
Mg |
Cu |
2.70 |
|
Mg |
Fe |
1.92 |
|
Zn |
Cu |
1.10 |
|
Fe |
Cu |
0.78 |
|
Zn |
Fe |
0.32 |
|
Cu |
Cu |
0.00 |
|
[6]
2. A fuel cell is an electrochemical cell in which the reactants are
supplied from outside (rather than forming an integral part of its
construction); a typical example, that of a hydrogen-oxygen fuel cell,
is shown in the diagram below.
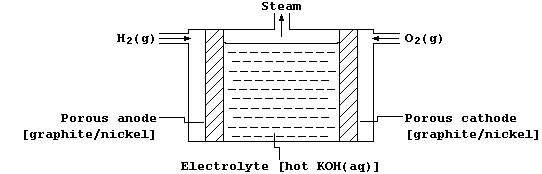
The overall reaction in this fuel cell is identical to the combustion
of dihydrogen; i.e.,
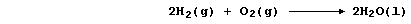
However, the oxidation and reduction are carried out separately at the
anode and cathode, respectively; i.e.,
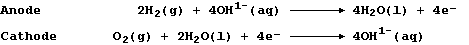
The graphite/nickel electrodes have two purposes: first, they serve as
electrical conductors; and second, they provide the necessary surfaces
for the dissociation of molecules into atoms, with nickel acting as an
electrocatalyst, prior to electron transfer.
(a) The symbol equation for the complete combustion of propane is:
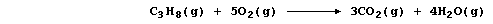
This redox reaction can be carried out in a fuel cell using platinum as
the electrocatalyst. Noting this symbol equation, and the equation for
the anodic reaction, construct the equation for the cathodic reaction.
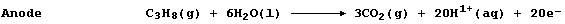
Cathode _______________________________________________________________
[2]
(b) Properly designed fuel cells are about 70% efficent and are free of
noise and thermal pollution. Despite these attractive features, fuel
cells are not yet in use for the large-scale generation of electrical
energy because of two unresolved (and related) difficulties: first, the
unavailability of suitable electrocatalysts able to function for long
periods of time without being 'poisoned'; and second, that of obtaining
a supply of sufficiently pure fuels. Suggest and explain how impurities
reduce the efficiency of such catalysts. ______________________________
_______________________________________________________________________
_______________________________________________________________________
[2]
_______________________________________________________________________
_______________________________________________________________________
Comedy-Dramas for Year 10-12 Students in British Orthography :
[No. 1] [No. 2] [No. 3] [No. 4] [No. 5] [No. 6] [No. 7] [No. 8] [No. 9]
[Dr. Roger Peters : rpeters@wissensdrang.com ; Aufbau1 ; Home Page]